The traditional antidiarrheal remedy, Garcinia buchananii stem bark extract, inhibits propulsive motility and fast synaptic potentials in the guinea pig distal colon
- PMID: 20718943
- PMCID: PMC2975827
- DOI: 10.1111/j.1365-2982.2010.01583.x
The traditional antidiarrheal remedy, Garcinia buchananii stem bark extract, inhibits propulsive motility and fast synaptic potentials in the guinea pig distal colon
Abstract
Background: Garcinia buchananii bark extract is a traditional African remedy for diarrhea, dysentery, abdominal discomfort, and pain. We investigated the mechanisms and efficacy of this extract using the guinea pig distal colon model of gastrointestinal motility.
Methods: Stem bark was collected from G. buchananii trees in their natural habitat of Karagwe, Tanzania. Bark was sun dried and ground into fine powder, and suspended in Krebs to obtain an aqueous extract. Isolated guinea pig distal colon was used to determine the effect of the G. buchananii bark extract on fecal pellet propulsion. Intracellular recording was used to evaluate the extract action on evoked fast excitatory postsynaptic potentials (fEPSPs) in S-neurons of the myenteric plexus.
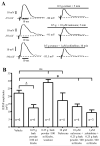
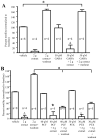
Key results: Garcinia buchananii bark extract inhibited pellet propulsion in a concentration-dependent manner, with an optimal concentration of ∼10 mg powder per mL Krebs. Interestingly, washout of the extract resulted in an increase in pellet propulsion to a level above basal activity. The extract reversibly reduced the amplitude of evoked fEPSPs in myenteric neurons. The extract's inhibitory action on propulsive motility and fEPSPs was not affected by the opioid receptor antagonist, naloxone, or the alpha- 2 adrenoceptor antagonist, yohimbine. The extract inhibited pellet motility in the presence of gamma-aminobutyric acid (GABA), GABA(A) and GABA(B) receptor antagonists picrotoxin and phaclofen, respectively. However, phaclofen and picrotoxin inhibited recovery rebound of motility during washout.
Conclusions & inferences: Garcinia buchananii extract has the potential to provide an effective, non-opiate antidiarrheal drug. Further studies are required to characterize bioactive components and elucidate the mechanisms of action, efficacy, and safety.
© 2010 Blackwell Publishing Ltd.
Figures


References
-
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365(9465):1147–52. - PubMed
-
- Nwachukwu CE, Okebe JU. Antimotility agents for chronic diarrhoea in people with HIV/AIDS. Cochrane Database Syst Rev. 2008;(4) CD005644. - PubMed
-
- Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet. 2004;363(9409):641–53. - PubMed
-
- UNICEF/WHO Diarrhoea: why children are still dying and what can be done WHO: New report 2009. 2009:1–68. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

