Trichostatin A and 5-azacytidine both cause an increase in global histone H4 acetylation and a decrease in global DNA and H3K9 methylation during mitosis in maize
- PMID: 20718950
- PMCID: PMC3095308
- DOI: 10.1186/1471-2229-10-178
Trichostatin A and 5-azacytidine both cause an increase in global histone H4 acetylation and a decrease in global DNA and H3K9 methylation during mitosis in maize
Abstract
Background: Modifications of DNA and histones in various combinations are correlated with many cellular processes. In this study, we investigated the possible relationship between histone H4 tetraacetylation, DNA methylation and histone H3 dimethylation at lysine 9 during mitosis in maize root meristems.
Results: Treatment with trichostatin A, which inhibits histone deacetylases, resulted in increased histone H4 acetylation accompanied by the decondensation of interphase chromatin and a decrease in both global H3K9 dimethylation and DNA methylation during mitosis in maize root tip cells. These observations suggest that histone acetylation may affect DNA and histone methylation during mitosis. Treatment with 5-azacytidine, a cytosine analog that reduces DNA methylation, caused chromatin decondensation and mediated an increase in H4 acetylation, in addition to reduced DNA methylation and H3K9 dimethylation during interphase and mitosis. These results suggest that decreased DNA methylation causes a reduction in H3K9 dimethylation and an increase in H4 acetylation.
Conclusions: The interchangeable effects of 5-azacytidine and trichostatin A on H4 acetylation, DNA methylation and H3K9 dimethylation indicate a mutually reinforcing action between histone acetylation, DNA methylation and histone methylation with respect to chromatin modification. Treatment with trichostatin A and 5-azacytidine treatment caused a decrease in the mitotic index, suggesting that H4 deacetylation and DNA and H3K9 methylation may contain the necessary information for triggering mitosis in maize root tips.
Figures

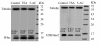




Similar articles
-
Analysis of specific lysine histone H3 and H4 acetylation and methylation status in clones of cells with a gene silenced by nickel exposure.Toxicol Appl Pharmacol. 2003 Aug 1;190(3):272-7. doi: 10.1016/s0041-008x(03)00169-8. Toxicol Appl Pharmacol. 2003. PMID: 12902198
-
Diverse histone modifications on histone 3 lysine 9 and their relation to DNA methylation in specifying gene silencing.BMC Genomics. 2007 May 24;8:131. doi: 10.1186/1471-2164-8-131. BMC Genomics. 2007. PMID: 17524140 Free PMC article.
-
Treatment of buffalo (Bubalus bubalis) donor cells with trichostatin A and 5-aza-2'-deoxycytidine alters their growth characteristics, gene expression and epigenetic status and improves the in vitro developmental competence, quality and epigenetic status of cloned embryos.Reprod Fertil Dev. 2016 Apr;28(6):824-37. doi: 10.1071/RD14176. Reprod Fertil Dev. 2016. PMID: 25409339
-
Reactivation of silenced genes and transcriptional therapy.Cytogenet Genome Res. 2003;100(1-4):56-64. doi: 10.1159/000072838. Cytogenet Genome Res. 2003. PMID: 14526164 Review.
-
Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases.Antioxid Redox Signal. 2013 May 20;18(15):1956-71. doi: 10.1089/ars.2012.4863. Epub 2012 Nov 6. Antioxid Redox Signal. 2013. PMID: 22978694 Free PMC article. Review.
Cited by
-
Trichostatin A Affects Developmental Reprogramming of Bread Wheat Microspores towards an Embryogenic Route.Plants (Basel). 2020 Oct 26;9(11):1442. doi: 10.3390/plants9111442. Plants (Basel). 2020. PMID: 33114625 Free PMC article.
-
AtEAF1 is a potential platform protein for Arabidopsis NuA4 acetyltransferase complex.BMC Plant Biol. 2015 Mar 5;15:75. doi: 10.1186/s12870-015-0461-1. BMC Plant Biol. 2015. PMID: 25849764 Free PMC article.
-
Trichostatin A and sodium butyrate promotes plant regeneration in common wheat.Plant Signal Behav. 2020 Dec 1;15(12):1820681. doi: 10.1080/15592324.2020.1820681. Epub 2020 Sep 22. Plant Signal Behav. 2020. PMID: 32962515 Free PMC article.
-
Trichostatin A increases embryo and green plant regeneration in wheat.Plant Cell Rep. 2017 Nov;36(11):1701-1706. doi: 10.1007/s00299-017-2183-3. Epub 2017 Jul 27. Plant Cell Rep. 2017. PMID: 28752355
-
Trichostatin A selectively suppresses the cold-induced transcription of the ZmDREB1 gene in maize.PLoS One. 2011;6(7):e22132. doi: 10.1371/journal.pone.0022132. Epub 2011 Jul 21. PLoS One. 2011. PMID: 21811564 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

