Imatinib induced severe skin reactions and neutropenia in a patient with gastrointestinal stromal tumor
- PMID: 20718969
- PMCID: PMC2936326
- DOI: 10.1186/1471-2407-10-438
Imatinib induced severe skin reactions and neutropenia in a patient with gastrointestinal stromal tumor
Abstract
Background: Imatinib mesylate has been used for the treatment of unresectable or metastatic gastrointestinal stromal tumors (GIST). The current recommended dose of imatinib is 400 mg/day that is increased to 800 mg/day in cases with disease progression. However, imatinib can be associated with diverse adverse events, which has limited its use. We report a case of severe adverse skin reactions with neutropenic fever during imatinib treatment in a patient with GIST.
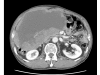
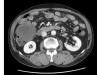
Case presentation: A 71-year-old man was admitted with a one month history of epigastric pain and a palpable mass in the right upper quadrant. An abdominal CT scan revealed a 20 x 19 cm intraabdominal mass with tumor invasion into the peritoneum. Needle biopsy was performed and the results showed spindle shaped tumor cells that were positive for c-KIT. The patient was diagnosed with unresectable GIST. Imatinib 400 mg/day was started. The patient tolerated the first eight weeks of treatment. However, about three months later, the patient developed a grade 4 febrile neutropenia and a grade 3 exfoliative skin rash. The patient recovered from this serious adverse events after discontinuation of imatinib with supportive care. However, the skin lesions recurred whenever the patient received imatinib over 100 mg/day. Therefore, imatinib 100 mg/day was maintained. Despite the low dose imatinib, follow up CT showed a marked partial response without grade 3 or 4 toxicities.
Conclusion: The recommended dose of imatinib for the treatment of GIST is 400 mg/day but patients at risk for adverse drug reaction may benefit from lower doses. Individualized treatment is needed for such patients, and we may also try sunitinib as a alternative drug.
Figures


Similar articles
-
Imatinib mesylate-induced acute hepatitis in a patient treated for gastrointestinal stromal tumour.Eur J Gastroenterol Hepatol. 2006 Jul;18(7):785-7. doi: 10.1097/01.meg.0000216941.42306.0e. Eur J Gastroenterol Hepatol. 2006. PMID: 16772838 Review.
-
Concurrent male gynecomastia and testicular hydrocele after imatinib mesylate treatment of a gastrointestinal stromal tumor.J Korean Med Sci. 2005 Jun;20(3):512-5. doi: 10.3346/jkms.2005.20.3.512. J Korean Med Sci. 2005. PMID: 15953881 Free PMC article.
-
A long-term follow-up of the imatinib mesylate treatment for the patients with recurrent gastrointestinal stromal tumor (GIST): the liver metastasis and the outcome.BMC Cancer. 2010 May 13;10:199. doi: 10.1186/1471-2407-10-199. BMC Cancer. 2010. PMID: 20465813 Free PMC article.
-
Efficacy and safety profile of imatinib mesylate (ST1571) in Japanese patients with advanced gastrointestinal stromal tumors: a phase II study (STI571B1202).Int J Clin Oncol. 2008 Jun;13(3):244-51. doi: 10.1007/s10147-007-0746-y. Epub 2008 Jun 14. Int J Clin Oncol. 2008. PMID: 18553235 Clinical Trial.
-
Gastrointestinal stromal tumor (GIST) and imatinib.Int J Clin Oncol. 2006 Jun;11(3):184-9. doi: 10.1007/s10147-006-0579-0. Int J Clin Oncol. 2006. PMID: 16850124 Review.
Cited by
-
Development and external validation of a nomogram for individualized adjuvant imatinib duration for high-risk gastrointestinal stromal tumors: A multicenter retrospective cohort study.Cancer Med. 2022 Aug;11(16):3093-3105. doi: 10.1002/cam4.4673. Epub 2022 Mar 16. Cancer Med. 2022. PMID: 35297216 Free PMC article.
-
Imatinib Desensitization After a Type IV Hypersensitivity Reaction in a Gastrointestinal Stromal Tumor Patient-A Case Report.Cancer Rep (Hoboken). 2025 Jun;8(6):e70238. doi: 10.1002/cnr2.70238. Cancer Rep (Hoboken). 2025. PMID: 40498648 Free PMC article.
-
Anti-apoptotic HAX-1 suppresses cell apoptosis by promoting c-Abl kinase-involved ROS clearance.Cell Death Dis. 2022 Apr 4;13(4):298. doi: 10.1038/s41419-022-04748-2. Cell Death Dis. 2022. PMID: 35379774 Free PMC article.
-
Discovery of an agonistic Siglec-6 antibody that inhibits and reduces human mast cells.Commun Biol. 2022 Nov 11;5(1):1226. doi: 10.1038/s42003-022-04207-w. Commun Biol. 2022. PMID: 36369358 Free PMC article.
-
Bullous Pemphigoid Occurring after Stopping Imatinib Therapy of CML: Is a Continuation of Post-Treatment Follow-Up Needed?Clin Pract. 2023 Sep 5;13(5):1082-1089. doi: 10.3390/clinpract13050096. Clin Pract. 2023. PMID: 37736932 Free PMC article.
References
-
- Zalcberg JR, Verweij J, Casali PG, Le Cesne A, Reichardt P, Blay JY, Schlemmer M, Van Glabbeke M, Brown M, Judson IR. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41:1751–1757. doi: 10.1016/j.ejca.2005.04.034. - DOI - PubMed
-
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. - DOI - PubMed
-
- Van Glabbeke M, Verweij J, Casali PG, Simes J, Le Cesne A, Reichardt P, Issels R, Judson IR, van Oosterom AT, Blay JY. Predicting toxicities for patients with advanced gastrointestinal stromal tumours treated with imatinib: a study of the European Organisation for Research and Treatment of Cancer, the Italian Sarcoma Group, and the Australasian Gastro-Intestinal Trials Group (EORTC-ISG-AGITG) Eur J Cancer. 2006;42:2277–2285. doi: 10.1016/j.ejca.2006.03.029. - DOI - PubMed
-
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228–231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

