Effect of nitrous oxide on cisatracurium infusion demands: a randomized controlled trial
- PMID: 20718983
- PMCID: PMC2931508
- DOI: 10.1186/1471-2253-10-14
Effect of nitrous oxide on cisatracurium infusion demands: a randomized controlled trial
Abstract
Background: Recent studies have questioned our previous understanding on the effect of nitrous oxide on muscle relaxants, since nitrous oxide has been shown to potentiate the action of bolus doses of mivacurium, rocuronium and vecuronium. This study was aimed to investigate the possible effect of nitrous oxide on the infusion requirements of cisatracurium.
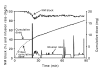
Methods: 70 ASA physical status I-III patients aged 18-75 years were enrolled in this randomized trial. The patients were undergoing elective surgery requiring general anesthesia with a duration of at least 90 minutes. Patients were randomized to receive propofol and remifentanil by target controlled infusion in combination with either a mixture of oxygen and nitrous oxide (Nitrous oxide/TIVA group) or oxygen in air (Air/TIVA group). A 0.1 mg/kg initial bolus of cisatracurium was administered before tracheal intubation, followed by a closed-loop computer controlled infusion of cisatracurium to produce and maintain a 90% neuromuscular block. Cumulative dose requirements of cisatracurium during the 90-min study period after bolus administration were measured and the asymptotic steady state rate of infusion to produce a constant 90% block was determined by applying nonlinear curve fitting to the data on the cumulative dose requirement during the study period.
Results: Controller performance, i.e. the ability of the controller to maintain neuromuscular block constant at the setpoint and patient characteristics were similar in both groups. The administration of nitrous oxide did not affect cisatracurium infusion requirements. The mean steady-state rates of infusion were 0.072 +/- 0.018 and 0.066 +/- 0.017 mg * kg-1 * h-1 in Air/TIVA and Nitrous oxide/TIVA groups, respectively.
Conclusions: Nitrous oxide does not affect the infusion requirements of cisatracurium.
Trial registration: ClinicalTrials.gov NCT01152905; European Clinical Trials Database at http://eudract.emea.eu.int/2006-006037-41.
Figures

References
-
- Eger EI. In: Miller's Anesthesia. 10. Miller RD, editor. Philadelphia: Elsevier Churchill Livingstone; 2005. Uptake and distribution; pp. 131–153.
Associated data
LinkOut - more resources
Full Text Sources
Medical

