The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition
- PMID: 20718984
- PMCID: PMC2933702
- DOI: 10.1186/1750-2187-5-14
The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition
Abstract
Background: Much attention has been recently focused on the role of cancer stem cells (CSCs) in the initiation and progression of solid malignancies. Since CSCs are able to proliferate and self-renew extensively due to their ability to express anti-apoptotic and drug resistant proteins, thus sustaining tumor growth. Therefore, the strategy to eradicate CSCs might have significant clinical implications. The objectives of this study were to examine the molecular mechanisms by which epigallocathechin gallate (EGCG) inhibits stem cell characteristics of prostate CSCs, and synergizes with quercetin, a major polyphenol and flavonoid commonly detected in many fruits and vegetables.
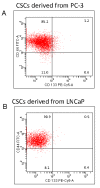
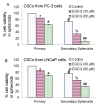
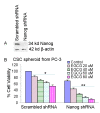
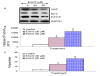
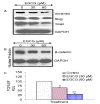
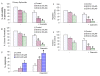
Results: Our data indicate that human prostate cancer cell lines contain a small population of CD44+CD133+ cancer stem cells and their self-renewal capacity is inhibited by EGCG. Furthermore, EGCG inhibits the self-renewal capacity of CD44+alpha2beta1+CD133+ CSCs isolated from human primary prostate tumors, as measured by spheroid formation in suspension. EGCG induces apoptosis by activating capase-3/7 and inhibiting the expression of Bcl-2, survivin and XIAP in CSCs. Furthermore, EGCG inhibits epithelial-mesenchymal transition by inhibiting the expression of vimentin, slug, snail and nuclear beta-catenin, and the activity of LEF-1/TCF responsive reporter, and also retards CSC's migration and invasion, suggesting the blockade of signaling involved in early metastasis. Interestingly, quercetin synergizes with EGCG in inhibiting the self-renewal properties of prostate CSCs, inducing apoptosis, and blocking CSC's migration and invasion. These data suggest that EGCG either alone or in combination with quercetin can eliminate cancer stem cell-characteristics.
Conclusion: Since carcinogenesis is a complex process, combination of bioactive dietary agents with complementary activities will be beneficial for prostate cancer prevention and/ortreatment.
Figures

References
-
- American. Cancer Society Cancer Statistics. 2009.
-
- Institute NC. Defeating prostate cancer: Crucial directions for research. Report of the Prostate Cancer Progress Review Group. 1998. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

