Delivery of a therapeutic protein by immune-privileged Sertoli cells
- PMID: 20719072
- PMCID: PMC3087628
- DOI: 10.3727/096368910X516628
Delivery of a therapeutic protein by immune-privileged Sertoli cells
Abstract
Immune-privileged Sertoli cells survive long term after allogeneic or xenogeneic transplantation without the use of immunosuppressive drugs, suggesting they could be used as a vehicle to deliver therapeutic proteins. As a model to test this, we engineered Sertoli cells to transiently produce basal levels of insulin and then examined their ability to lower blood glucose levels after transplantation into diabetic SCID mice. Mouse and porcine Sertoli cells transduced with a recombinant adenoviral vector containing furin-modified human proinsulin cDNA expressed insulin mRNA and secreted insulin protein. Transplantation of 5-20 million insulin-expressing porcine Sertoli cells into diabetic SCID mice significantly decreased blood glucose levels in a dose-dependent manner, with 20 million Sertoli cells decreasing blood glucose levels to 9.8 ± 2.7 mM. Similar results were obtained when 20 million insulin-positive, BALB/c mouse Sertoli cells were transplanted; blood glucose levels dropped to 6.3 ± 2.4 mM and remained significantly lower for 5 days. To our knowledge, this is the first study to demonstrate Sertoli cells can be engineered to produce and secrete a clinically relevant factor that has a therapeutic effect, thus supporting the concept of using immune-privileged Sertoli cells as a potential vehicle for gene therapy.
Figures

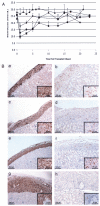
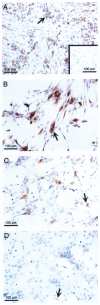

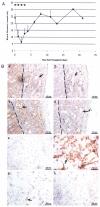
References
-
- Barry SC, Ramesh N, Lejnieks D, Simonson WT, Kemper L, Lernmark A, Osborne WR. Glucose-regulated insulin expression in diabetic rats. Hum. Gene Ther. 2001;12(2):131–139. - PubMed
-
- Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377(6550):630–632. - PubMed
-
- Davidson HW. (Pro)Insulin processing: A historical perspective. Cell Biochem. Biophys. 2004;40(3 Suppl.):143–158. - PubMed
-
- Dong H, Altomonte J, Morral N, Meseck M, Thung SN, Woo SL. Basal insulin gene expression significantly improves conventional insulin therapy in type 1 diabetic rats. Diabetes. 2002;51(1):130–138. - PubMed
-
- Dufour JM, Dass B, Halley KR, Korbutt GS, Dixon DE, Rajotte RV. Sertoli cell line lacks the immunoprotective properties associated with primary Sertoli cells. Cell Transplant. 2008;17(5):525–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

