Direct, real-time measurement of shear stress-induced nitric oxide produced from endothelial cells in vitro
- PMID: 20719252
- PMCID: PMC2965060
- DOI: 10.1016/j.niox.2010.08.003
Direct, real-time measurement of shear stress-induced nitric oxide produced from endothelial cells in vitro
Abstract
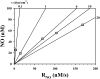
Nitric oxide (NO) produced by the endothelium is involved in the regulation of vascular tone. Decreased NO production or availability has been linked to endothelial dysfunction in hypercholesterolemia and hypertension. Shear stress-induced NO release is a well-established phenomenon, yet the cellular mechanisms of this response are not completely understood. Experimental limitations have hindered direct, real-time measurements of NO under flow conditions. We have overcome these challenges with a new design for a parallel-plate flow chamber. The chamber consists of two compartments, separated by a Transwell® membrane, which isolates a NO recording electrode located in the upper compartment from flow effects. Endothelial cells are grown on the bottom of the membrane, which is inserted into the chamber flush with the upper plate. We demonstrate for the first time direct real-time NO measurements from endothelial cells with controlled variations in shear stress. Step changes in shear stress from 0.1 dyn/cm(2) to 6, 10, or 20 dyn/cm(2) elicited a transient decrease in NO followed by an increase to a new steady state. An analysis of NO transport suggests that the initial decrease is due to the increased removal rate by convection as flow increases. Furthermore, the rate at which the NO concentration approaches the new steady state is related to the time-dependent cellular response rather than transport limitations of the measurement configuration. Our design offers a method for studying the kinetics of the signaling mechanisms linking NO production with shear stress as well as pathological conditions involving changes in NO production or availability.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
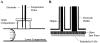












References
-
- Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear-stress induced release of nitricoxide from endothelial-cells grown on beads. Hypertension. 1991;17:187–193. - PubMed
-
- Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–8. - PubMed
-
- Radomski MW, Vallance P, Whitley G, Foxwell N, Moncada S. Platelet adhesion to human vascular endothelium is modulated by constitutive and cytokine induced nitric oxide. Cardiovasc Res. 1993;27:1380–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

