Phosphorylation-state-dependent regulation of NMDA receptor short-term plasticity modifies hippocampal dendritic Ca2+ transients
- PMID: 20719921
- PMCID: PMC2957449
- DOI: 10.1152/jn.01081.2009
Phosphorylation-state-dependent regulation of NMDA receptor short-term plasticity modifies hippocampal dendritic Ca2+ transients
Abstract
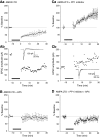
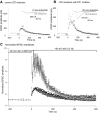
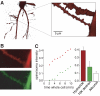
N-methyl-D-aspartate (NMDA) receptor-mediated currents are enhanced by phosphorylation. We have investigated effects of phosphorylation-dependent short-term plasticity of NMDA receptor-mediated excitatory postsynaptic currents (EPSCs) on the induction of long-term depression (LTD). We confirmed in whole cell clamped CA1 pyramidal neurons that LTD is induced by pairing stimulus protocols. However, after serine-threonine phosphorylation was modified by postsynaptic introduction of a protein phosphatase-1 (PP1) inhibitor, the same pairing protocol evoked long-term potentiation (LTP). We determined effects of modification of phosphatase activity on evoked NMDA EPSCs during LTD induction protocols. During LTD induction, using a protocol pairing depolarization to -40 mV and 0.5 Hz stimulation, NMDA receptor-mediated EPSCs undergo a short-term enhancement at the start of the protocol. In neurons in which PP1 activity was inhibited, this short-term enhancement was markedly amplified. We then investigated the effect of this enhancement on Ca(2+) entry during the start of the LTD induction protocol. Enhancement of NMDA receptor-mediated responses was accompanied by an amplification of induction protocol-evoked Ca(2+) transients. Furthermore, this amplification required synaptic activation during the protocol, consistent with an enhancement of Ca(2+) entry mediated by NMDA receptor activation. The sign of NMDA receptor-mediated long-term plasticity, whether potentiation or depression depends on the amplitude of the synaptic Ca(2+) transient during induction. We conclude that short-term phosphorylation-dependent plasticity of the NMDA receptor-mediated EPSCs contributes significantly to the effect of phosphatase inhibition on the subsequent induction of LTD or LTP.
Figures
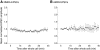


Similar articles
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
-
Distinct trafficking and expression mechanisms underlie LTP and LTD of NMDA receptor-mediated synaptic responses.Hippocampus. 2010 May;20(5):646-58. doi: 10.1002/hipo.20654. Hippocampus. 2010. PMID: 19489005
-
A model of NMDA receptor-mediated activity in dendrites of hippocampal CA1 pyramidal neurons.J Neurophysiol. 1992 Dec;68(6):2248-59. doi: 10.1152/jn.1992.68.6.2248. J Neurophysiol. 1992. PMID: 1337105 Free PMC article.
-
Differential induction of LTP and LTD is not determined solely by instantaneous calcium concentration: an essential involvement of a temporal factor.Eur J Neurosci. 2001 Aug;14(4):701-8. doi: 10.1046/j.0953-816x.2001.01679.x. Eur J Neurosci. 2001. PMID: 11556894
-
Muscarinic Receptors, from Synaptic Plasticity to its Role in Network Activity.Neuroscience. 2021 Feb 21;456:60-70. doi: 10.1016/j.neuroscience.2020.04.005. Epub 2020 Apr 8. Neuroscience. 2021. PMID: 32278062 Review.
Cited by
-
Separate intramolecular targets for protein kinase A control N-methyl-D-aspartate receptor gating and Ca2+ permeability.J Biol Chem. 2014 Jul 4;289(27):18805-17. doi: 10.1074/jbc.M113.537282. Epub 2014 May 20. J Biol Chem. 2014. PMID: 24847051 Free PMC article.
-
Calcineurin Controls Hypothalamic NMDA Receptor Activity and Sympathetic Outflow.Circ Res. 2022 Aug 5;131(4):345-360. doi: 10.1161/CIRCRESAHA.122.320976. Epub 2022 Jul 15. Circ Res. 2022. PMID: 35862168 Free PMC article.
-
Endogenous casein kinase-1 modulates NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension.J Physiol. 2015 Oct 1;593(19):4439-52. doi: 10.1113/JP270831. Epub 2015 Aug 18. J Physiol. 2015. PMID: 26174743 Free PMC article.
References
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126–130, 1996 - PubMed
-
- Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature 349: 156–158, 1991 - PubMed
-
- Bear MF. Mechanism for a sliding synaptic modification threshold. Neuron 15: 1–4, 1995 - PubMed
-
- Bortolotto ZA, Collett VJ, Conquet F, Jia Z, van der Putten H, Collingridge GL. The regulation of hippocampal LTP by the molecular switch, a form of metaplasticity, requires mGlu5 receptors. Neuropharmacology 49, Suppl 1: 13–25, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

