Molecular layer inhibitory interneurons provide feedforward and lateral inhibition in the dorsal cochlear nucleus
- PMID: 20719922
- PMCID: PMC2997026
- DOI: 10.1152/jn.00312.2010
Molecular layer inhibitory interneurons provide feedforward and lateral inhibition in the dorsal cochlear nucleus
Abstract
In the outer layers of the dorsal cochlear nucleus, a cerebellum-like structure in the auditory brain stem, multimodal sensory inputs drive parallel fibers to excite both principal (fusiform) cells and inhibitory cartwheel cells. Cartwheel cells, in turn, inhibit fusiform cells and other cartwheel cells. At the microcircuit level, it is unknown how these circuit components interact to modulate the activity of fusiform cells and thereby shape the processing of auditory information. Using a variety of approaches in mouse brain stem slices, we investigated the synaptic connectivity and synaptic strength among parallel fibers, cartwheel cells, and fusiform cells. In paired recordings of spontaneous and evoked activity, we found little overlap in parallel fiber input to neighboring neurons, and activation of multiple parallel fibers was required to evoke or alter action potential firing in cartwheel and fusiform cells. Thus neighboring neurons likely respond best to distinct subsets of sensory inputs. In contrast, there was significant overlap in inhibitory input to neighboring neurons. In recordings from synaptically coupled pairs, cartwheel cells had a high probability of synapsing onto nearby fusiform cells or other nearby cartwheel cells. Moreover, single cartwheel cells strongly inhibited spontaneous firing in single fusiform cells. These synaptic relationships suggest that the set of parallel fibers activated by a particular sensory stimulus determines whether cartwheel cells provide feedforward or lateral inhibition to their postsynaptic targets.
Figures
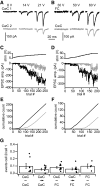

 ) were skewed toward larger events than distributions of mEPSC amplitudes (■). In cartwheel cells, mean EPSC amplitudes were 22.4 ± 2.8, mEPSCs, and 39.5 ± 21.0, sEPSCs. In fusiform cells, mean EPSC amplitudes were 21.6 ± 4.0, mEPSCs, and 46.3 ± 51.0, sEPSCs. E and F: cumulative distributions of the amplitude data shown in C and D, respectively. More than 99% of mEPSCs were smaller than the amplitude cutoffs (dashed lines), which were established to distinguish between sEPSCs that might have resulted from miniature release events and those that were probably the product of action potential-evoked release. In cartwheel cells, 11% of sEPSCs were larger than the 74 pA cutoff, and, in fusiform cells, 13% were larger than the 65 pA cutoff. G and H: cumulative distributions of instantaneous EPSC frequencies. Mean sEPSC frequencies were significantly higher than mean mEPSC frequencies in cartwheel cells, G, and fusiform cells, H, (t-test, P < 0.05). Cartwheel cells: sEPSC frequency = 29 ± 9 Hz, mEPSC frequency = 14 ± 5 Hz. Fusiform cells: sEPSC frequency = 33 ± 9 Hz, mEPSC frequency = 20 ± 3 Hz. All distributions represent averages of distributions across multiple cells for each group. Cartwheel cells: n = 8, mEPSC group; 52, sEPSC group. Fusiform cells: n = 6, mEPSC group; 22, sEPSC group.
) were skewed toward larger events than distributions of mEPSC amplitudes (■). In cartwheel cells, mean EPSC amplitudes were 22.4 ± 2.8, mEPSCs, and 39.5 ± 21.0, sEPSCs. In fusiform cells, mean EPSC amplitudes were 21.6 ± 4.0, mEPSCs, and 46.3 ± 51.0, sEPSCs. E and F: cumulative distributions of the amplitude data shown in C and D, respectively. More than 99% of mEPSCs were smaller than the amplitude cutoffs (dashed lines), which were established to distinguish between sEPSCs that might have resulted from miniature release events and those that were probably the product of action potential-evoked release. In cartwheel cells, 11% of sEPSCs were larger than the 74 pA cutoff, and, in fusiform cells, 13% were larger than the 65 pA cutoff. G and H: cumulative distributions of instantaneous EPSC frequencies. Mean sEPSC frequencies were significantly higher than mean mEPSC frequencies in cartwheel cells, G, and fusiform cells, H, (t-test, P < 0.05). Cartwheel cells: sEPSC frequency = 29 ± 9 Hz, mEPSC frequency = 14 ± 5 Hz. Fusiform cells: sEPSC frequency = 33 ± 9 Hz, mEPSC frequency = 20 ± 3 Hz. All distributions represent averages of distributions across multiple cells for each group. Cartwheel cells: n = 8, mEPSC group; 52, sEPSC group. Fusiform cells: n = 6, mEPSC group; 22, sEPSC group.



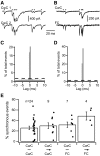
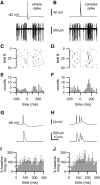
References
-
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol 82: 1697–1709, 1999 - PubMed
-
- Balakrishnan V, Trussell LO. Synaptic inputs to granule cells of the dorsal cochlear nucleus. J Neurophysiol 99: 208–219, 2008 - PubMed
-
- Barbour B. Synaptic currents evoked in Purkinje cells by stimulating individual granule cells. Neuron 11: 759–769, 1993 - PubMed
-
- Berrebi AS, Mugnaini E. Distribution and targets of the cartwheel cell axon in the dorsal cochlear nucleus of the guinea pig. Anat Embryol 183: 427–454, 1991 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

