Successful treatment of melanoma brain metastases with adoptive cell therapy
- PMID: 20719934
- PMCID: PMC6291850
- DOI: 10.1158/1078-0432.CCR-10-1507
Successful treatment of melanoma brain metastases with adoptive cell therapy
Abstract
Purpose: To determine the objective response rate and response duration of melanoma brain metastases to adoptive cell therapy (ACT) with autologous antitumor lymphocytes plus interleukin-2 following a lymphodepleting preparative regimen.
Methods: Between 2000 and 2009, 264 patients with metastatic melanoma received ACT, consisting of cyclophosphamide and fludarabine with or without total body irradiation, followed by the infusion of autologous tumor-infiltrating lymphocytes (TIL) or autologous peripheral blood lymphocytes retrovirally transduced to express a T-cell receptor (TCR) that recognized the melanocyte differentiation antigens gp-100 or MART-1. From this group, 26 patients were retrospectively identified to have had untreated brain metastases and extracranial disease before receiving ACT. The response rate and duration of melanoma brain metastases, as well as the overall response rate, response duration, and survival for these patients, are presented.
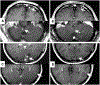
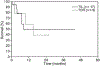
Results: Seventeen of these 26 patients received ACT with TIL. Seven of these patients (41%) achieved a complete response in the brain, and six patients achieved an overall partial response. In the nine patients that received TCR-transduced lymphocytes, two patients achieved a complete response in the brain (22%) and one of these two achieved an overall partial response. One patient developed a tumor-associated subarachnoid hemorrhage during the thrombocytopenic phase of therapy and had an uneventful metastatectomy.
Conclusion: ACT with a nonmyeloablative preparative regimen using either TIL- or TCR gene-transduced cells and interleukin-2 can mediate complete and durable regression of melanoma brain metastases. This strategy can be used safely in selected patients with metastatic melanoma to the brain.
©2010 AACR.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures

References
-
- Harrison BE, Johnson JL, Clough RW, Halperin EC. Selection of patients with melanoma brain metastases for aggressive treatment. Am J Clin Oncol 2003;26:354–7. - PubMed
-
- Sampson JH, Carter JH, Jr., Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg 1998;88:11–20. - PubMed
-
- Gaudy-Marqueste C, Regis JM, Muracciole X, et al. Gamma-knife radiosurgery in the management of melanoma patients with brain metastases: a series of 106 patients without whole-brain radiotherapy. Int J Radiat Oncol Biol Phys 2006;65:809–16. - PubMed
-
- Seung SK, Sneed PK, McDermott MW, et al. Gamma knife radiosurgery for malignant melanoma brain metastases. Cancer J Sci Am 1998;4:103–9. - PubMed
-
- Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol 2004;22:2101–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

