Swine influenza H1N1 virus induces acute inflammatory immune responses in pig lungs: a potential animal model for human H1N1 influenza virus
- PMID: 20719941
- PMCID: PMC2953174
- DOI: 10.1128/JVI.01211-10
Swine influenza H1N1 virus induces acute inflammatory immune responses in pig lungs: a potential animal model for human H1N1 influenza virus
Abstract
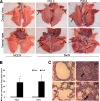
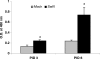
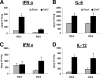
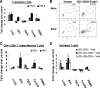
Pigs are capable of generating reassortant influenza viruses of pandemic potential, as both the avian and mammalian influenza viruses can infect pig epithelial cells in the respiratory tract. The source of the current influenza pandemic is H1N1 influenza A virus, possibly of swine origin. This study was conducted to understand better the pathogenesis of H1N1 influenza virus and associated host mucosal immune responses during acute infection in humans. Therefore, we chose a H1N1 swine influenza virus, Sw/OH/24366/07 (SwIV), which has a history of transmission to humans. Clinically, inoculated pigs had nasal discharge and fever and shed virus through nasal secretions. Like pandemic H1N1, SwIV also replicated extensively in both the upper and lower respiratory tracts, and lung lesions were typical of H1N1 infection. We detected innate, proinflammatory, Th1, Th2, and Th3 cytokines, as well as SwIV-specific IgA antibody in lungs of the virus-inoculated pigs. Production of IFN-γ by lymphocytes of the tracheobronchial lymph nodes was also detected. Higher frequencies of cytotoxic T lymphocytes, γδ T cells, dendritic cells, activated T cells, and CD4+ and CD8+ T cells were detected in SwIV-infected pig lungs. Concomitantly, higher frequencies of the immunosuppressive T regulatory cells were also detected in the virus-infected pig lungs. The findings of this study have relevance to pathogenesis of the pandemic H1N1 influenza virus in humans; thus, pigs may serve as a useful animal model to design and test effective mucosal vaccines and therapeutics against influenza virus.
Figures


References
-
- Reference deleted.
-
- Basta, S., C. P. Carrasco, S. M. Knoetig, R. C. Rigden, H. Gerber, A. Summerfield, and K. C. McCullough. 2000. Porcine alveolar macrophages: poor accessory or effective suppressor cells for T-lymphocytes. Vet. Immunol. Immunopathol. 77:177-190. - PubMed
-
- Brookes, S. M., A. Nunez, B. Choudhury, M. Matrosovich, S. C. Essen, D. Clifford, M. J. Slomka, G. Kuntz-Simon, F. Garcon, B. Nash, A. Hanna, P. M. Heegaard, S. Queguiner, C. Chiapponi, M. Bublot, J. M. Garcia, R. Gardner, E. Foni, W. Loeffen, L. Larsen, K. Van Reeth, J. Banks, R. M. Irvine, and I. H. Brown. 2010. Replication, pathogenesis and transmission of pandemic (H1N1) 2009 virus in non-immune pigs. PLoS One 5:e9068. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

