The F gene of the Osaka-2 strain of measles virus derived from a case of subacute sclerosing panencephalitis is a major determinant of neurovirulence
- PMID: 20719945
- PMCID: PMC2953189
- DOI: 10.1128/JVI.01075-10
The F gene of the Osaka-2 strain of measles virus derived from a case of subacute sclerosing panencephalitis is a major determinant of neurovirulence
Abstract
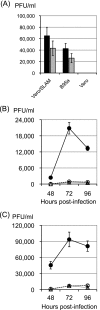
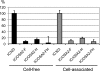
Measles virus (MV) is the causative agent for acute measles and subacute sclerosing panencephalitis (SSPE). Although numerous mutations have been found in the MV genome of SSPE strains, the mutations responsible for the neurovirulence have not been determined. We previously reported that the SSPE Osaka-2 strain but not the wild-type strains of MV induced acute encephalopathy when they were inoculated intracerebrally into 3-week-old hamsters. The recombinant MV system was adapted for the current study to identify the gene(s) responsible for neurovirulence in our hamster model. Recombinant viruses that contained envelope-associated genes from the Osaka-2 strain were generated on the IC323 wild-type MV background. The recombinant virus containing the M gene alone did not induce neurological disease, whereas the H gene partially contributed to neurovirulence. In sharp contrast, the recombinant virus containing the F gene alone induced lethal encephalopathy. This phenotype was related to the ability of the F protein to induce syncytium formation in Vero cells. Further study indicated that a single T461I substitution in the F protein was sufficient to transform the nonneuropathogenic wild-type MV into a lethal virus for hamsters.
Figures


References
-
- Albrecht, P., and H. P. Schumacher. 1971. Neurotropic properties of measles virus in hamsters and mice. J. Infect. Dis. 124:86-93. - PubMed
-
- Albrecht, P., T. Burnstein, M. J. Klutch, H. T. Hicks, and F. A. Ennis. 1977. Subacute sclerosing panencephalitis: experimental infection in primates. Science 195:64-66. - PubMed
-
- Ayata, M., K. Hayashi, T. Seto, R. Murata, and H. Ogura. 1998. The matrix gene expression of subacute sclerosing panencephalitis (SSPE) virus (Osaka-1 strain): a comparison of two sibling viruses isolated from different lobes of an SSPE brain. Microbiol. Immunol. 42:773-780. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

