Poxvirus complement control proteins are expressed on the cell surface through an intermolecular disulfide bridge with the viral A56 protein
- PMID: 20719953
- PMCID: PMC2953161
- DOI: 10.1128/JVI.00372-10
Poxvirus complement control proteins are expressed on the cell surface through an intermolecular disulfide bridge with the viral A56 protein
Abstract
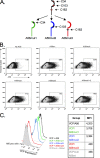
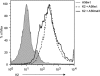
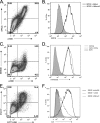
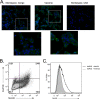
The vaccinia virus (VACV) complement control protein (VCP) is an immunomodulatory protein that is both secreted from and expressed on the surface of infected cells. Surface expression of VCP occurs though an interaction with the viral transmembrane protein A56 and is dependent on a free N-terminal cysteine of VCP. Although A56 and VCP have been shown to interact in infected cells, the mechanism remains unclear. To investigate if A56 is sufficient for surface expression, we transiently expressed VCP and A56 in eukaryotic cell lines and found that they interact on the cell surface in the absence of other viral proteins. Since A56 contains three extracellular cysteines, we hypothesized that one of the cysteines may be unpaired and could therefore form a disulfide bridge with VCP. To test this, we generated a series of A56 mutants in which each cysteine was mutated to a serine, and we found that mutation of cysteine 162 abrogated VCP cell surface expression. We also tested the ability of other poxvirus complement control proteins to bind to VACV A56. While the smallpox homolog of VCP is able to bind VACV A56, the ectromelia virus (ECTV) VCP homolog is only able to bind the ECTV homolog of A56, indicating that these proteins may have coevolved. Surface expression of poxvirus complement control proteins may have important implications in viral pathogenesis, as a virus that does not express cell surface VCP is attenuated in vivo. This suggests that surface expression of VCP may contribute to poxvirus pathogenesis.
Figures



References
-
- Brum, L. M., P. C. Turner, H. Devick, M. T. Baquero, and R. W. Moyer. 2003. Plasma membrane localization and fusion inhibitory activity of the cowpox virus serpin SPI-3 require a functional signal sequence and the virus encoded hemagglutinin. Virology 306:289-302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

