Role of the Rhesus glycoprotein, Rh B glycoprotein, in renal ammonia excretion
- PMID: 20719974
- PMCID: PMC2980396
- DOI: 10.1152/ajprenal.00277.2010
Role of the Rhesus glycoprotein, Rh B glycoprotein, in renal ammonia excretion
Abstract
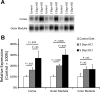
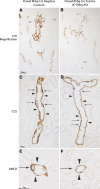
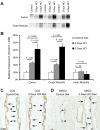
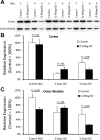
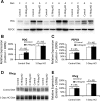
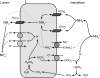
Rh B glycoprotein (Rhbg) is a member of the Rh glycoprotein family of ammonia transporters. In the current study, we examine Rhbg's role in basal and acidosis-stimulated acid-base homeostasis. Metabolic acidosis induced by HCl administration increased Rhbg expression in both the cortex and outer medulla. To test the functional significance of increased Rhbg expression, we used a Cre-loxP approach to generate mice with intercalated cell-specific Rhbg knockout (IC-Rhbg-KO). On normal diet, intercalated cell-specific Rhbg deletion did not alter urine ammonia excretion, pH, or titratable acid excretion significantly, but it did decrease glutamine synthetase expression in the outer medulla significantly. After metabolic acidosis was induced, urinary ammonia excretion was significantly less in IC-Rhbg-KO than in control (C) mice on days 2-4 of acid loading, but not on day 5. Urine pH and titratable acid excretion and dietary acid intake did not differ significantly between acid-loaded IC-Rhcg-KO and C mice. In IC-Rhbg-KO mice, acid loading increased connecting segment (CNT) cell and outer medullary collecting duct principal cell Rhbg expression. In both C and IC-Rhbg-KO mice, acid loading decreased glutamine synthetase in both the cortex and outer medulla; the decrease on day 3 was similar in IC-Rhbg-KO and C mice, but on day 5 it was significantly greater in IC-Rhbg-KO than in C mice. We conclude 1) intercalated cell Rhbg contributes to acidosis-stimulated renal ammonia excretion, 2) Rhbg in CNT and principal cells may contribute to renal ammonia excretion, and 3) decreased glutamine synthetase expression may enable normal rates of ammonia excretion under both basal conditions and on day 5 of acid loading in IC-Rhbg-KO mice.
Figures



References
-
- Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 - PubMed
-
- Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, Leviel F, Geoffroy V, Cambillau M, Colin Y, Paillard M, Houillier P, Cartron JP, Eladari D. Genetic ablation of Rhbg in mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol 289: F1281–F1290, 2005 - PubMed
-
- Conjard A, Komaty O, Delage H, Boghossian M, Martin M, Ferrier B, Baverel G. Inhibition of glutamine synthetase in the mouse kidney: a novel mechanism of adaptation to metabolic acidosis. J Biol Chem 278: 38159–38166, 2003 - PubMed
-
- Damian AC, Pitts RF. Rates of glutaminase I and glutamine synthetase reactions in rat kidney in vivo. Am J Physiol 218: 1249–1255, 1970 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

