Hyperaldosteronism in Klotho-deficient mice
- PMID: 20719979
- PMCID: PMC3774497
- DOI: 10.1152/ajprenal.00233.2010
Hyperaldosteronism in Klotho-deficient mice
Abstract
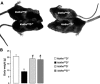
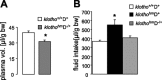
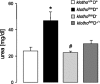
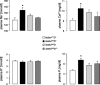
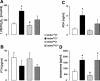
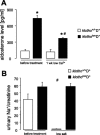
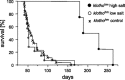
Klotho is a membrane protein participating in the inhibitory effect of FGF23 on the formation of 1,25-dihydroxyvitamin-D(3) [1,25(OH)(2)D(3)]. It participates in the regulation of renal tubular phosphate reabsorption and stimulates renal tubular Ca(2+) reabsorption. Klotho hypomorphic mice (klotho(hm)) suffer from severe growth deficit, rapid aging, and early death, events largely reversed by a vitamin D-deficient diet. The present study explored the role of Klotho deficiency in mineral and electrolyte metabolism. To this end, klotho(hm) mice and wild-type mice (klotho(+/+)) were subjected to a normal (D(+)) or vitamin D-deficient (D(-)) diet or to a vitamin D-deficient diet for 4 wk and then to a normal diet (D(-/+)). At the age of 8 wk, body weight was significantly lower in klotho(hm)D(+) mice than in klotho(+/+)D(+) mice, klotho(hm)D(-) mice, and klotho(hm)D(-/+) mice. Plasma concentrations of 1,25(OH)(2)D(3,) adrenocorticotropic hormone (ACTH), antidiuretic hormone (ADH), and aldosterone were significantly higher in klotho(hm)D(+) mice than in klotho(+/+)D(+) mice. Plasma volume was significantly smaller in klotho(hm)D(-/+) mice, and plasma urea, Ca(2+), phosphate and Na(+), but not K(+) concentrations were significantly higher in klotho(hm)D(+) mice than in klotho(+/+)D(+) mice. The differences were partially abrogated by a vitamin D-deficient diet. Moreover, the hyperaldosteronism was partially reversed by Ca(2+)-deficient diet. Ussing chamber experiments revealed a marked increase in amiloride-sensitive current across the colonic epithelium, pointing to enhanced epithelial sodium channel (ENaC) activity. A salt-deficient diet tended to decrease and a salt-rich diet significantly increased the life span of klotho(hm)D(+) mice. In conclusion, the present observation disclose that the excessive formation of 1,25(OH)(2)D(3) in Klotho-deficient mice results in extracellular volume depletion, which significantly contributes to the shortening of life span.
Figures


References
-
- Berger S, Bleich M, Schmid W, Greger R, Schutz G. Mineralocorticoid receptor knockout mice: lessons on Na+ metabolism. Kidney Int 57: 1295–1298, 2000 - PubMed
-
- Foldager N, Blomqvist CG. Repeated plasma volume determination with the Evans Blue dye dilution technique: the method and a computer program. Comput Biol Med 21: 35–41, 1991 - PubMed
-
- Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004 - PubMed
-
- Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y. Alpha-Klotho as a regulator of calcium homeostasis. Science 316: 1615–1618, 2007 - PubMed
-
- Kempe DS, Ackermann TF, Fischer SS, Koka S, Boini KM, Mahmud H, Föller M, Rosenblatt KP, Kuro-o M, Lang F. Accelerated suicidal erythrocyte death in Klotho-deficient mice. Pflügers Arch 458: 503–512, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

