Shear stress increases nitric oxide production in thick ascending limbs
- PMID: 20719980
- PMCID: PMC2980401
- DOI: 10.1152/ajprenal.00112.2010
Shear stress increases nitric oxide production in thick ascending limbs
Abstract
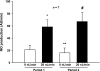
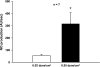
We showed that luminal flow stimulates nitric oxide (NO) production in thick ascending limbs. Ion delivery, stretch, pressure, and shear stress all increase when flow is enhanced. We hypothesized that shear stress stimulates NO in thick ascending limbs, whereas stretch, pressure, and ion delivery do not. We measured NO in isolated, perfused rat thick ascending limbs using the NO-sensitive dye DAF FM-DA. NO production rose from 21 ± 7 to 58 ± 12 AU/min (P < 0.02; n = 7) when we increased luminal flow from 0 to 20 nl/min, but dropped to 16 ± 8 AU/min (P < 0.02; n = 7) 10 min after flow was stopped. Flow did not increase NO in tubules from mice lacking NO synthase 3 (NOS 3). Flow stimulated NO production by the same extent in tubules perfused with ion-free solution and physiological saline (20 ± 7 vs. 24 ± 6 AU/min; n = 7). Increasing stretch while reducing shear stress and pressure lowered NO generation from 42 ± 9 to 17 ± 6 AU/min (P < 0.03; n = 6). In the absence of shear stress, increasing pressure and stretch had no effect on NO production (2 ± 8 vs. 8 ± 8 AU/min; n = 6). Similar results were obtained in the presence of tempol (100 μmol/l), a O(2)(-) scavenger. Primary cultures of thick ascending limb cells subjected to shear stresses of 0.02 and 0.55 dyne/cm(2) produced NO at rates of 55 ± 10 and 315 ± 93 AU/s, respectively (P < 0.002; n = 7). Pretreatment with the NOS inhibitor l-NAME (5 mmol/l) blocked the shear stress-induced increase in NO production. We concluded that shear stress rather than pressure, stretch, or ion delivery mediates flow-induced stimulation of NO by NOS 3 in thick ascending limbs.
Figures





References
-
- Baer PG, Bianchi G, Liliana D. Renal micropuncture study of normotensive and Milan hypertensive rats before and after development of hypertension. Kidney Int 13: 452–466, 1978 - PubMed
-
- Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 277: 3388–3396, 2002 - PubMed
-
- Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension 17: 187–193, 1991 - PubMed
-
- Cai Z, Xin J, Pollock DM, Pollock JS. Shear stress-mediated NO production in inner medullary collecting duct cells. Am J Physiol Renal Physiol 279: F270–F274, 2000 - PubMed
-
- Danciu TE, Adam RM, Naruse K, Freeman MR, Hauschka PV. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett 536: 193–197, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

