Direct demonstration of tubular fluid flow sensing by macula densa cells
- PMID: 20719981
- PMCID: PMC2980403
- DOI: 10.1152/ajprenal.00469.2009
Direct demonstration of tubular fluid flow sensing by macula densa cells
Abstract
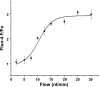
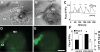
Macula densa (MD) cells in the cortical thick ascending limb (cTAL) detect variations in tubular fluid composition and transmit signals to the afferent arteriole (AA) that control glomerular filtration rate [tubuloglomerular feedback (TGF)]. Increases in tubular salt at the MD that normally parallel elevations in tubular fluid flow rate are well accepted as the trigger of TGF. The present study aimed to test whether MD cells can detect variations in tubular fluid flow rate per se. Calcium imaging of the in vitro microperfused isolated JGA-glomerulus complex dissected from mice was performed using fluo-4 and fluorescence microscopy. Increasing cTAL flow from 2 to 20 nl/min (80 mM [NaCl]) rapidly produced significant elevations in cytosolic Ca(2+) concentration ([Ca(2+)](i)) in AA smooth muscle cells [evidenced by changes in fluo-4 intensity (F); F/F(0) = 1.45 ± 0.11] and AA vasoconstriction. Complete removal of the cTAL around the MD plaque and application of laminar flow through a perfusion pipette directly to the MD apical surface essentially produced the same results even when low (10 mM) or zero NaCl solutions were used. Acetylated α-tubulin immunohistochemistry identified the presence of primary cilia in mouse MD cells. Under no flow conditions, bending MD cilia directly with a micropipette rapidly caused significant [Ca(2+)](i) elevations in AA smooth muscle cells (fluo-4 F/F(0): 1.60 ± 0.12) and vasoconstriction. P2 receptor blockade with suramin significantly reduced the flow-induced TGF, whereas scavenging superoxide with tempol did not. In conclusion, MD cells are equipped with a tubular flow-sensing mechanism that may contribute to MD cell function and TGF.
Figures

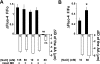


Similar articles
-
Calcium wave of tubuloglomerular feedback.Am J Physiol Renal Physiol. 2006 Aug;291(2):F473-80. doi: 10.1152/ajprenal.00425.2005. Epub 2006 Feb 21. Am J Physiol Renal Physiol. 2006. PMID: 16495210
-
Dynamic aspects of the tubuloglomerular feedback mechanism.Dan Med Bull. 1992 Apr;39(2):134-54. Dan Med Bull. 1992. PMID: 1611920 Review.
-
Fluid flow in the juxtaglomerular interstitium visualized in vivo.Am J Physiol Renal Physiol. 2006 Dec;291(6):F1241-7. doi: 10.1152/ajprenal.00203.2006. Epub 2006 Jul 25. Am J Physiol Renal Physiol. 2006. PMID: 16868308
-
Possible mechanism of efferent arteriole (Ef-Art) tubuloglomerular feedback.Kidney Int. 2007 May;71(9):861-6. doi: 10.1038/sj.ki.5002161. Epub 2007 Mar 7. Kidney Int. 2007. PMID: 17342182
-
Cellular mechanisms within the juxtaglomerular apparatus.Am J Hypertens. 1990 Jan;3(1):76-80. doi: 10.1093/ajh/3.1.76. Am J Hypertens. 1990. PMID: 2405885 Review.
Cited by
-
Stretch-Induced Increases in Intracellular Ca Stimulate Thick Ascending Limb O2- Production and Are Enhanced in Dahl Salt-Sensitive Rats.Hypertension. 2020 Feb;75(2):431-438. doi: 10.1161/HYPERTENSIONAHA.119.13765. Epub 2019 Dec 23. Hypertension. 2020. PMID: 31865796 Free PMC article.
-
Maintained tubuloglomerular feedback responses during acute inhibition of P2 purinergic receptors in mice.Am J Physiol Renal Physiol. 2011 Feb;300(2):F339-44. doi: 10.1152/ajprenal.00637.2010. Epub 2010 Dec 8. Am J Physiol Renal Physiol. 2011. PMID: 21147842 Free PMC article.
-
Sensing a sensor: identifying the mechanosensory function of primary cilia.Biosensors (Basel). 2014 Mar;4(1):47-62. doi: 10.3390/bios4010047. Biosensors (Basel). 2014. PMID: 24839551 Free PMC article.
-
A new view of macula densa cell protein synthesis.Am J Physiol Renal Physiol. 2021 Dec 1;321(6):F689-F704. doi: 10.1152/ajprenal.00222.2021. Epub 2021 Oct 25. Am J Physiol Renal Physiol. 2021. PMID: 34693742 Free PMC article.
-
Ovine uterine space restriction causes dysregulation of the renin-angiotensin system in fetal kidneys.Biol Reprod. 2017 Jan 1;96(1):211-220. doi: 10.1095/biolreprod.116.140079. Biol Reprod. 2017. PMID: 28395333 Free PMC article.
References
-
- Bell PD, McLean CB, Navar LG. Dissociation of tubuloglomerular feedback responses from distal tubular chloride concentration in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 240: F111–F119, 1981 - PubMed
-
- Bell PB, Navar LG. Relationship between tubulo-glomerulat feedback responses and perfusate hypotonicity. Kidney Int 22: 234–239, 1982 - PubMed
-
- Briggs J, Schubert G, Schnermann J. Further evidence for an inverse relationship between macula densa NaCl concentration and filtration rate. Pflügers Arch 392: 372–378, 1982 - PubMed
-
- Carlström M, Persson AE. Important role of NAD(P)H oxidase 2 in the regulation of the tubuloglomerular feedback. Hypertension 53: 456–457, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

