Identification of Mycobacterium tuberculosis antigens of high serodiagnostic value
- PMID: 20719988
- PMCID: PMC2952993
- DOI: 10.1128/CVI.00198-10
Identification of Mycobacterium tuberculosis antigens of high serodiagnostic value
Abstract
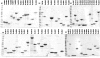
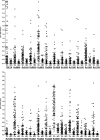
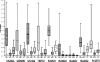
Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis, with several million new cases detected each year. Current methods of diagnosis are time-consuming and/or expensive or have a low level of accuracy. Therefore, new diagnostics are urgently needed to address the global tuberculosis burden and to improve control programs. Serological assays remain attractive for use in resource-limited settings because they are simple, rapid, and inexpensive and offer the possibility of detecting cases often missed by routine sputum smear microscopy. The aim of this study was to identify M. tuberculosis seroreactive antigens from a panel of 103 recombinant proteins selected as diagnostic candidates. Initial library screening by protein array analysis and enzyme-linked immunosorbent assay (ELISA) identified 42 antigens with serodiagnostic potential. Among these, 25 were novel proteins. The reactive antigens demonstrated various individual sensitivities, ranging from 12% to 78% (specificities, 76 to 100%). When the antigens were analyzed in combinations, up to 93% of antibody responders could be identified among the TB patients. Selected seroreactive proteins were used to design 3 new polyepitope fusion proteins. Characterization of these antigens by multiantigen print immunoassay (MAPIA) revealed that the vast majority of the TB patients (90%) produced antibody responses. The results confirmed that due to the remarkable variation in immune recognition patterns, an optimal multiantigen cocktail should be designed to cover the heterogeneity of antibody responses and thus achieve the highest possible test sensitivity.
Figures

Similar articles
-
Identification of Novel Antigens Recognized by Serum Antibodies in Bovine Tuberculosis.Clin Vaccine Immunol. 2017 Dec 5;24(12):e00259-17. doi: 10.1128/CVI.00259-17. Print 2017 Dec. Clin Vaccine Immunol. 2017. PMID: 28978510 Free PMC article.
-
Mycobacterium tuberculosis lipolytic enzymes as potential biomarkers for the diagnosis of active tuberculosis.PLoS One. 2011;6(9):e25078. doi: 10.1371/journal.pone.0025078. Epub 2011 Sep 22. PLoS One. 2011. PMID: 21966416 Free PMC article.
-
Humoral immune responses against the Mycobacterium tuberculosis 38-kilodalton, MTB48, and CFP-10/ESAT-6 antigens in tuberculosis.Clin Vaccine Immunol. 2010 Mar;17(3):372-5. doi: 10.1128/CVI.00287-09. Epub 2010 Jan 6. Clin Vaccine Immunol. 2010. PMID: 20053875 Free PMC article.
-
Antibody responses to mycobacterial antigens in children with tuberculosis: challenges and potential diagnostic value.Clin Vaccine Immunol. 2012 Dec;19(12):1898-906. doi: 10.1128/CVI.00501-12. Epub 2012 Oct 24. Clin Vaccine Immunol. 2012. PMID: 23100476 Free PMC article. Review.
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
Cited by
-
emPAI-assisted strategy enhances screening and assessment of Mycobacterium tuberculosis infection serological markers.Microb Biotechnol. 2021 Jul;14(4):1827-1838. doi: 10.1111/1751-7915.13829. Epub 2021 Jun 26. Microb Biotechnol. 2021. PMID: 34173722 Free PMC article.
-
Identification of Antibody Targets for Tuberculosis Serology using High-Density Nucleic Acid Programmable Protein Arrays.Mol Cell Proteomics. 2017 Apr;16(4 suppl 1):S277-S289. doi: 10.1074/mcp.M116.065953. Epub 2017 Feb 21. Mol Cell Proteomics. 2017. PMID: 28223349 Free PMC article.
-
The role of antibodies in tuberculosis diagnosis, prophylaxis and therapy: a review from the ESGMYC study group.Eur Respir Rev. 2022 Mar 9;31(163):210218. doi: 10.1183/16000617.0218-2021. Print 2022 Mar 31. Eur Respir Rev. 2022. PMID: 35264411 Free PMC article. Review.
-
Role of B cells and antibodies in acquired immunity against Mycobacterium tuberculosis.Cold Spring Harb Perspect Med. 2014 Oct 9;5(3):a018432. doi: 10.1101/cshperspect.a018432. Cold Spring Harb Perspect Med. 2014. PMID: 25301934 Free PMC article. Review.
-
A multicentric evaluation of the recombinant Leishmania infantum antigen-based immunochromatographic assay for the serodiagnosis of canine visceral leishmaniasis.Parasit Vectors. 2014 Mar 31;7:136. doi: 10.1186/1756-3305-7-136. Parasit Vectors. 2014. PMID: 24684857 Free PMC article.
References
-
- Abebe, F., C. Holm-Hansen, H. G. Wiker, and G. Bjune. 2007. Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scand. J. Immunol. 66:176-191. - PubMed
-
- Al Zahrani, K., H. Al Jahdali, L. Poirier, P. Rene, M. L. Gennaro, and D. Menzies. 2000. Accuracy and utility of commercially available amplification and serologic tests for the diagnosis of minimal pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 162:1323-1329. - PubMed
-
- Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. - PubMed
-
- Bertholet, S., G. C. Ireton, M. Kahn, J. Guderian, R. Mohamath, N. Stride, E. M. Laughlin, S. L. Baldwin, T. S. Vedvick, R. N. Coler, and S. G. Reed. 2008. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J. Immunol. 181:7948-7957. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

