TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+
- PMID: 20720007
- PMCID: PMC2966118
- DOI: 10.1074/jbc.M110.156927
TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+
Abstract
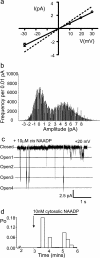
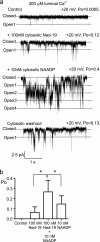
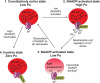
Nicotinic acid adenine dinucleotide phosphate (NAADP) is a molecule capable of initiating the release of intracellular Ca(2+) required for many essential cellular processes. Recent evidence links two-pore channels (TPCs) with NAADP-induced release of Ca(2+) from lysosome-like acidic organelles; however, there has been no direct demonstration that TPCs can act as NAADP-sensitive Ca(2+) release channels. Controversial evidence also proposes ryanodine receptors as the primary target of NAADP. We show that TPC2, the major lysosomal targeted isoform, is a cation channel with selectivity for Ca(2+) that will enable it to act as a Ca(2+) release channel in the cellular environment. NAADP opens TPC2 channels in a concentration-dependent manner, binding to high affinity activation and low affinity inhibition sites. At the core of this process is the luminal environment of the channel. The sensitivity of TPC2 to NAADP is steeply dependent on the luminal [Ca(2+)] allowing extremely low levels of NAADP to open the channel. In parallel, luminal pH controls NAADP affinity for TPC2 by switching from reversible activation of TPC2 at low pH to irreversible activation at neutral pH. Further evidence earmarking TPCs as the likely pathway for NAADP-induced intracellular Ca(2+) release is obtained from the use of Ned-19, the selective blocker of cellular NAADP-induced Ca(2+) release. Ned-19 antagonizes NAADP-activation of TPC2 in a non-competitive manner at 1 μM but potentiates NAADP activation at nanomolar concentrations. This single-channel study provides a long awaited molecular basis for the peculiar mechanistic features of NAADP signaling and a framework for understanding how NAADP can mediate key physiological events.
Figures






References
-
- Lee H. C., Aarhus R. (1995) J. Biol. Chem. 270, 2152–2157 - PubMed
-
- Lee H. C. (2000) J. Membr. Biol. 173, 1–8 - PubMed
-
- Genazzani A. A., Galione A. (1997) Trends Pharmacol. Sci. 18, 108–110 - PubMed
-
- Guse A. H. (2009) Curr. Biol. 19, R521–R523 - PubMed
-
- Patel S., Churchill G. C., Galione A. (2001) Trends Biochem. Sci. 26, 482–489 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

