Factors from human embryonic stem cell-derived fibroblast-like cells promote topology-dependent hepatic differentiation in primate embryonic and induced pluripotent stem cells
- PMID: 20720011
- PMCID: PMC2963358
- DOI: 10.1074/jbc.M110.122093
Factors from human embryonic stem cell-derived fibroblast-like cells promote topology-dependent hepatic differentiation in primate embryonic and induced pluripotent stem cells
Abstract
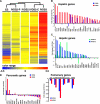
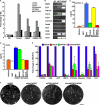
The future clinical use of embryonic stem cell (ESC)-based hepatocyte replacement therapy depends on the development of an efficient procedure for differentiation of hepatocytes from ESCs. Here we report that a high density of human ESC-derived fibroblast-like cells (hESdFs) supported the efficient generation of hepatocyte-like cells with functional and mature hepatic phenotypes from primate ESCs and human induced pluripotent stem cells. Molecular and immunocytochemistry analyses revealed that hESdFs caused a rapid loss of pluripotency and induced a sequential endoderm-to-hepatocyte differentiation in the central area of ESC colonies. Knockdown experiments demonstrated that pluripotent stem cells were directed toward endodermal and hepatic lineages by FGF2 and activin A secreted from hESdFs. Furthermore, we found that the central region of ESC colonies was essential for the hepatic endoderm-specific differentiation, because its removal caused a complete disruption of endodermal differentiation. In conclusion, we describe a novel in vitro differentiation model and show that hESdF-secreted factors act in concert with regional features of ESC colonies to induce robust hepatic endoderm differentiation in primate pluripotent stem cells.
Figures





References
-
- Akhter J., Johnson L. A., Gunasegaram A., Riordan S. M., Morris D. L. (2007) Surgeon 5, 155–164 - PubMed
-
- Mitalipov S., Kuo H. C., Byrne J., Clepper L., Meisner L., Johnson J., Zeier R., Wolf D. (2006) Stem Cells 24, 2177–2186 - PubMed
-
- Suemori H., Tada T., Torii R., Hosoi Y., Kobayashi K., Imahie H., Kondo Y., Iritani A., Nakatsuji N. (2001) Dev. Dyn. 222, 273–279 - PubMed
-
- Reubinoff B. E., Pera M. F., Fong C. Y., Trounson A., Bongso A. (2000) Nat. Biotechnol. 18, 399–404 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

