γ-Tocotrienol but not γ-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents
- PMID: 20720018
- PMCID: PMC2963373
- DOI: 10.1074/jbc.M110.158378
γ-Tocotrienol but not γ-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents
Retraction in
-
γ-Tocotrienol but not γ-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents.J Biol Chem. 2016 Aug 5;291(32):16922. doi: 10.1074/jbc.A110.158378. J Biol Chem. 2016. PMID: 27496963 Free PMC article. No abstract available.
Abstract
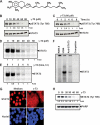
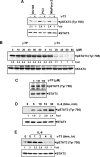
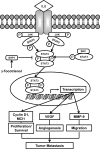
Although γ-tocotrienol (T3), a vitamin E isolated primarily from palm and rice bran oil, has been linked with anticancer activities, the mechanism of this action is poorly understood. In this study, we investigated whether γ-T3 can modulate the STAT3 cell signaling pathway, closely linked to inflammation and tumorigenesis. We found that γ-T3 but not γ-tocopherol, the most common saturated form of vitamin E, inhibited constitutive activation of STAT3 in a dose- and time-dependent manner, and this inhibition was not cell type-specific. γ-T3 also inhibited STAT3 DNA binding. This correlated with inhibition of Src kinase and JAK1 and JAK2 kinases. Pervanadate reversed the γ-T3-induced down-regulation of STAT3 activation, suggesting the involvement of a protein-tyrosine phosphatase. When examined further, we found that γ-T3 induced the expression of the tyrosine phosphatase SHP-1, and gene silencing of the SHP-1 by small interfering RNA abolished the ability of γ-T3 to inhibit STAT3 activation, suggesting a vital role for SHP-1 in the action of γ-T3. Also γ-T3 down-modulated activation of STAT3 and induced SHP-1 in vivo. Eventually, γ-T3 down-regulated the expression of STAT3-regulated antiapoptotic (Bcl-2, Bcl-xL, and Mcl-1), proliferative (cyclin D1), and angiogenic (VEGF) gene products; and this correlated with suppression of proliferation, the accumulation of cells in sub-G(1) phase of the cell cycle, and induction of apoptosis. This vitamin also sensitized the tumor cells to the apoptotic effects of thalidomide and bortezomib. Overall, our results suggest that γ-T3 is a novel blocker of STAT3 activation pathway both in vitro and in vivo and thus may have potential in prevention and treatment of cancers.
Figures



Similar articles
-
γ-Tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: potential role as an antiproliferative, pro-apoptotic and chemosensitizing agent.Br J Pharmacol. 2011 May;163(2):283-98. doi: 10.1111/j.1476-5381.2010.01187.x. Br J Pharmacol. 2011. PMID: 21198544 Free PMC article.
-
Betulinic acid suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase SHP-1 in human multiple myeloma cells.Int J Cancer. 2010 Jul 15;127(2):282-92. doi: 10.1002/ijc.25059. Int J Cancer. 2010. PMID: 19937797 Free PMC article.
-
Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation.Clin Cancer Res. 2007 May 15;13(10):3024-32. doi: 10.1158/1078-0432.CCR-06-2575. Clin Cancer Res. 2007. PMID: 17505005
-
Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy.Adv Nutr. 2017 Nov 15;8(6):850-867. doi: 10.3945/an.117.016329. Print 2017 Nov. Adv Nutr. 2017. PMID: 29141970 Free PMC article. Review.
-
How vitamin E and its derivatives regulate tumour cells via the MAPK signalling pathway?'.Gene. 2022 Jan 15;808:145998. doi: 10.1016/j.gene.2021.145998. Epub 2021 Oct 7. Gene. 2022. PMID: 34626718 Review.
Cited by
-
Prolonged survival and delayed progression of pancreatic intraepithelial neoplasia in LSL-KrasG12D/+;Pdx-1-Cre mice by vitamin E δ-tocotrienol.Carcinogenesis. 2013 Apr;34(4):858-63. doi: 10.1093/carcin/bgt002. Epub 2013 Jan 9. Carcinogenesis. 2013. PMID: 23302291 Free PMC article.
-
Turmeric (Curcuma longa) inhibits inflammatory nuclear factor (NF)-κB and NF-κB-regulated gene products and induces death receptors leading to suppressed proliferation, induced chemosensitization, and suppressed osteoclastogenesis.Mol Nutr Food Res. 2012 Mar;56(3):454-65. doi: 10.1002/mnfr.201100270. Epub 2011 Dec 7. Mol Nutr Food Res. 2012. PMID: 22147524 Free PMC article.
-
Antioxidant and Anti-Tumor Effects of Dietary Vitamins A, C, and E.Antioxidants (Basel). 2023 Mar 3;12(3):632. doi: 10.3390/antiox12030632. Antioxidants (Basel). 2023. PMID: 36978880 Free PMC article. Review.
-
Retraction notice to “Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake” [Biochem. Pharmacol. 80 (2010) 1021–1032].Biochem Pharmacol. 2016 Feb 15;102:142. doi: 10.1016/j.bcp.2015.11.007. Epub 2016 Feb 20. Biochem Pharmacol. 2016. PMID: 26985464 Free PMC article. No abstract available.
-
Tocotrienols fight cancer by targeting multiple cell signaling pathways.Genes Nutr. 2012 Jan;7(1):43-52. doi: 10.1007/s12263-011-0220-3. Epub 2011 Apr 9. Genes Nutr. 2012. PMID: 21484157 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

