Nuclear-localized calcineurin homologous protein CHP1 interacts with upstream binding factor and inhibits ribosomal RNA synthesis
- PMID: 20720019
- PMCID: PMC2978553
- DOI: 10.1074/jbc.M110.165555
Nuclear-localized calcineurin homologous protein CHP1 interacts with upstream binding factor and inhibits ribosomal RNA synthesis
Abstract
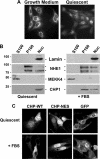
Calcineurin homologous protein 1 (CHP1) is a widely expressed, 22-kDa myristoylated EF-hand Ca(2+)-binding protein that shares a high degree of similarity with the regulatory B subunit of calcineurin (65%) and with calmodulin (59%). CHP1 localizes to the plasma membrane, the Golgi apparatus, and the nucleus and functions to regulate trafficking of early secretory vesicles, activation of T cells, and expression and transport of the Na-H exchanger NHE1. Although CHP1 contains nuclear export signals, whether its nuclear and cytoplasmic localization is regulated and has distinct functions remain unknown. We show that CHP1 is predominantly in the nucleus in quiescent fibroblasts, is translocated to cytoplasmic compartments with growth medium, and that translocation is inhibited by mutations in the nuclear export motifs. In a screen for proteins co-precipitating with CHP1 in quiescent cells we identified the upstream binding factor UBF, a DNA-binding protein and component of the RNA polymerase I complex regulating RNA synthesis. The CHP1-UBF interaction is restricted to the nucleus and inhibited by Ca(2+). Nuclear retention of CHP1 attenuates the abundance of UBF in the nucleolus and inhibits RNA synthesis when quiescent cells are transferred to growth medium. These data show UBF as a newly identified CHP1-binding protein and regulation of RNA synthesis as a newly identified function for nuclear-localized CHP1, which is distinct from CHP1 functions in the cytosol.
Figures




Similar articles
-
Calcineurin homologous protein: a multifunctional Ca2+-binding protein family.Am J Physiol Renal Physiol. 2012 Jul 15;303(2):F165-79. doi: 10.1152/ajprenal.00628.2011. Epub 2011 Dec 21. Am J Physiol Renal Physiol. 2012. PMID: 22189947 Free PMC article. Review.
-
Dual functional significance of calcineurin homologous protein 1 binding to Na(+)/H(+) exchanger isoform 1.Am J Physiol Cell Physiol. 2011 Aug;301(2):C280-8. doi: 10.1152/ajpcell.00404.2010. Epub 2011 May 4. Am J Physiol Cell Physiol. 2011. PMID: 21543739
-
Role of calcineurin B homologous protein in pH regulation by the Na+/H+ exchanger 1: tightly bound Ca2+ ions as important structural elements.Biochemistry. 2004 Mar 30;43(12):3628-36. doi: 10.1021/bi0360004. Biochemistry. 2004. PMID: 15035633
-
Two nuclear export signals specify the cytoplasmic localization of calcineurin B homologous protein 1.J Biochem. 2003 Dec;134(6):919-25. doi: 10.1093/jb/mvg223. J Biochem. 2003. PMID: 14769882
-
A role for upstream binding factor in organizing ribosomal gene chromatin.Biochem Soc Symp. 2006;(73):77-84. doi: 10.1042/bss0730077. Biochem Soc Symp. 2006. PMID: 16626289 Review.
Cited by
-
Plasma protein profiling in a stage defined pancreatic cancer cohort - Implications for early diagnosis.Mol Oncol. 2016 Oct;10(8):1305-16. doi: 10.1016/j.molonc.2016.07.001. Epub 2016 Jul 12. Mol Oncol. 2016. PMID: 27522951 Free PMC article.
-
Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus.Mol Microbiol. 2011 Dec;82(5):1235-59. doi: 10.1111/j.1365-2958.2011.07886.x. Epub 2011 Nov 8. Mol Microbiol. 2011. PMID: 22066998 Free PMC article.
-
Emerging roles of the single EF-hand Ca2+ sensor tescalcin in the regulation of gene expression, cell growth and differentiation.J Cell Sci. 2016 Oct 1;129(19):3533-3540. doi: 10.1242/jcs.191486. Epub 2016 Sep 8. J Cell Sci. 2016. PMID: 27609838 Free PMC article. Review.
-
Effect of huanglian jiedu decoction on thoracic aorta gene expression in spontaneous hypertensive rats.Evid Based Complement Alternat Med. 2014;2014:565784. doi: 10.1155/2014/565784. Epub 2014 Mar 13. Evid Based Complement Alternat Med. 2014. PMID: 24744811 Free PMC article.
-
Calcineurin homologous protein: a multifunctional Ca2+-binding protein family.Am J Physiol Renal Physiol. 2012 Jul 15;303(2):F165-79. doi: 10.1152/ajprenal.00628.2011. Epub 2011 Dec 21. Am J Physiol Renal Physiol. 2012. PMID: 22189947 Free PMC article. Review.
References
-
- Pang T., Su X., Wakabayashi S., Shigekawa M. (2001) J. Biol. Chem. 276, 17367–17372 - PubMed
-
- Pang T., Hisamitsu T., Mori H., Shigekawa M., Wakabayashi S. (2004) Biochemistry 43, 3628–3636 - PubMed
-
- Matsushita M., Sano Y., Yokoyama S., Takai T., Inoue H., Mitsui K., Todo K., Ohmori H., Kanazawa H. (2007) Am. J. Physiol. Cell Physiol. 293, C246–C254 - PubMed
-
- Di Sole F., Cerull R., Babich V., Quiñones H., Gisler S. M., Biber J., Murer H., Burckhardt G., Helmle-Kolb C., Moe O. W. (2004) J. Biol. Chem. 279, 2962–2974 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

