Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings
- PMID: 20720033
- PMCID: PMC2953088
- DOI: 10.1128/JCM.01053-10
Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings
Abstract
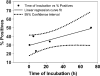
The recently introduced Xpert MTB/RIF assay (Xpert) has point-of-care potential, but its capacity for biohazard containment remained to be studied. We compared the bioaerosols generated by the Xpert assay to acid-fast bacillus (AFB) microscope slide smear preparation. The Xpert assay sample treatment reagent (SR) was also studied for its sterilizing capacity, stability, and effect on assay sensitivity after prolonged treatment. During the preparation of AFB smears, sputum samples spiked with Mycobacterium bovis BCG at 5 × 10(8) CFU/ml produced 16 and 325 CFU/m(3) air measured with an Andersen impactor or BioSampler, respectively. In contrast, neither the sample preparation steps for the Xpert assay nor its automated processing produced any culturable bioaerosols. In testing of SR sterilizing capacity, clinical sputum samples from strongly smear-positive tuberculosis patients treated with SR at a 2:1 ratio eliminated Mycobacterium tuberculosis growth in all but 1/39 or 3/45 samples cultured on solid or liquid medium, respectively. These few unsterilized samples had a mean 13.1-day delay in the time to positive culture. SR treatment at a 3:1 ratio eliminated growth in all samples. SR retained a greater than 6-log-unit killing capacity despite storage at temperatures spanning 4 to 45°C for at least 3 months. The effect of prolonged SR sample treatment was also studied. Spiked sputum samples could be incubated in SR for up to 3 days without affecting Xpert sensitivity for M. tuberculosis detection and up to 8 h without affecting specificity for rifampin resistance detection. These results suggest that benchtop use of the Xpert MTB/RIF assay limits infection risk to the user.
Figures



References
-
- Ad Hoc Committee for the Guidelines for Preventing the Transmission of Tuberculosis in Canadian Health Care Facilities and Other Institutional Settings. 1996. Guidelines for preventing the transmission of tuberculosis in Canadian health care facilities and other institutional settings. Can. Commun. Dis. Rep. 22(Suppl. 1):i-iv, 1-55. - PubMed
-
- An, H. R., G. Mainelis, and M. Yao. 2004. Evaluation of a high-volume portable bioaerosol sampler in laboratory and field environments. Indoor Air 14:385-393. - PubMed
-
- Association for the Advancement of Medical Instrumentation. 2003. Sterilization of health care products—requirements for products labeled “sterile.” Standard ANSI/AAMI ST67. Association for the Advancement of Medical Instrumentation, Arlington, VA.
-
- Bonnet, M., A. Ramsay, W. Githui, L. Gagnidze, F. Varaine, and P. J. Guerin. 2008. Bleach sedimentation: an opportunity to optimize smear microscopy for tuberculosis diagnosis in settings of high prevalence of HIV. Clin. Infect. Dis. 46:1710-1716. - PubMed
-
- Caulfield, J. L., J. S. Wishnok, and S. R. Tannenbaum. 1998. Nitric oxide-induced deamination of cytosine and guanine in deoxynucleosides and oligonucleotides. J. Biol. Chem. 273:12689-12695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

