Ectopic Reelin induces neuronal aggregation with a normal birthdate-dependent "inside-out" alignment in the developing neocortex
- PMID: 20720102
- PMCID: PMC6633482
- DOI: 10.1523/JNEUROSCI.0486-10.2010
Ectopic Reelin induces neuronal aggregation with a normal birthdate-dependent "inside-out" alignment in the developing neocortex
Abstract
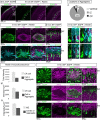
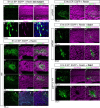
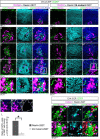
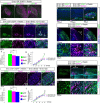
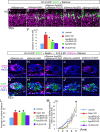
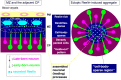
Neurons in the developing mammalian neocortex form the cortical plate (CP) in an "inside-out" manner; that is, earlier-born neurons form the deeper layers, whereas later-born neurons migrate past the existing layers and form the more superficial layers. Reelin, a glycoprotein secreted by Cajal-Retzius neurons in the marginal zone (MZ), is crucial for this "inside-out" layering, because the layers are inverted in the Reelin-deficient mouse, reeler (Reln(rl)). Even though more than a decade has passed since the discovery of reelin, the biological effect of Reelin on individual migrating neurons remains unclear. In addition, although the MZ is missing in the reeler cortex, it is unknown whether Reelin directly regulates the development of the cell-body-sparse MZ. To address these issues, we expressed Reelin ectopically in the developing mouse cortex, and the results showed that Reelin caused the leading processes of migrating neurons to assemble in the Reelin-rich region, which in turn induced their cell bodies to form cellular aggregates around Reelin. Interestingly, the ectopic Reelin-rich region became cell-body-sparse and dendrite-rich, resembling the MZ, and the late-born neurons migrated past their predecessors toward the central Reelin-rich region within the aggregates, resulting in a birthdate-dependent "inside-out" alignment even ectopically. Reelin receptors and intracellular adaptor protein Dab1 were found to be necessary for formation of the aggregates. The above findings indicate that Reelin signaling is capable of inducing the formation of the dendrite-rich, cell-body-sparse MZ and a birthdate-dependent "inside-out" alignment of neurons independently of other factors/structures near the MZ.
Figures

Similar articles
-
Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin α5β1.Neuron. 2012 Oct 18;76(2):353-69. doi: 10.1016/j.neuron.2012.07.020. Epub 2012 Oct 17. Neuron. 2012. PMID: 23083738 Free PMC article.
-
VLDLR is not essential for reelin-induced neuronal aggregation but suppresses neuronal invasion into the marginal zone.Development. 2020 Jun 15;147(12):dev189936. doi: 10.1242/dev.189936. Development. 2020. PMID: 32540847
-
Neurons tend to stop migration and differentiate along the cortical internal plexiform zones in the Reelin signal-deficient mice.J Neurosci Res. 2002 Sep 15;69(6):723-30. doi: 10.1002/jnr.10345. J Neurosci Res. 2002. PMID: 12205665
-
[Role of Reelin during Layer Formation in the Cerebral Neocortex].Brain Nerve. 2016 Aug;68(8):931-7. doi: 10.11477/mf.1416200532. Brain Nerve. 2016. PMID: 27503821 Review. Japanese.
-
How does Reelin control neuronal migration and layer formation in the developing mammalian neocortex?Neurosci Res. 2014 Sep;86:50-8. doi: 10.1016/j.neures.2014.06.004. Epub 2014 Jun 23. Neurosci Res. 2014. PMID: 24969097 Review.
Cited by
-
Reelin-Nrp1 Interaction Regulates Neocortical Dendrite Development in a Context-Specific Manner.J Neurosci. 2020 Oct 21;40(43):8248-8261. doi: 10.1523/JNEUROSCI.1907-20.2020. Epub 2020 Oct 2. J Neurosci. 2020. PMID: 33009002 Free PMC article.
-
De novo monoallelic Reelin missense variants cause dominant neuronal migration disorders via a dominant-negative mechanism.J Clin Invest. 2024 Jul 9;134(16):e153097. doi: 10.1172/JCI153097. J Clin Invest. 2024. PMID: 38980724 Free PMC article.
-
Reelin transiently promotes N-cadherin-dependent neuronal adhesion during mouse cortical development.Proc Natl Acad Sci U S A. 2017 Feb 21;114(8):2048-2053. doi: 10.1073/pnas.1615215114. Epub 2017 Feb 7. Proc Natl Acad Sci U S A. 2017. PMID: 28174271 Free PMC article.
-
Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin α5β1.Neuron. 2012 Oct 18;76(2):353-69. doi: 10.1016/j.neuron.2012.07.020. Epub 2012 Oct 17. Neuron. 2012. PMID: 23083738 Free PMC article.
-
Schizophrenia and Depression Co-Morbidity: What We have Learned from Animal Models.Front Psychiatry. 2015 Feb 18;6:13. doi: 10.3389/fpsyt.2015.00013. eCollection 2015. Front Psychiatry. 2015. PMID: 25762938 Free PMC article. Review.
References
-
- Ajioka I, Nakajima K. Birth-date-dependent segregation of the mouse cerebral cortical neurons in reaggregation cultures. Eur J Neurosci. 2005;22:331–342. - PubMed
-
- Bayer SA, Altman J. Neocortical development. New York: Raven; 1991.
-
- Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. - PubMed
-
- Caviness VS, Jr, Sidman RL. Time of origin or corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol. 1973;148:141–151. - PubMed
-
- Cooper JA. A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 2008;31:113–119. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
