The alpha-syntrophin PH and PDZ domains scaffold acetylcholine receptors, utrophin, and neuronal nitric oxide synthase at the neuromuscular junction
- PMID: 20720107
- PMCID: PMC2936458
- DOI: 10.1523/JNEUROSCI.1930-10.2010
The alpha-syntrophin PH and PDZ domains scaffold acetylcholine receptors, utrophin, and neuronal nitric oxide synthase at the neuromuscular junction
Abstract
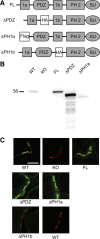
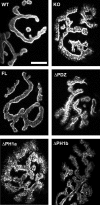
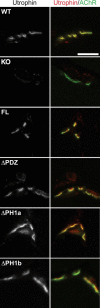
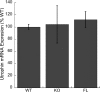
At the neuromuscular junction (NMJ), the dystrophin protein complex provides a scaffold that functions to stabilize acetylcholine receptor (AChR) clusters. Syntrophin, a key component of that scaffold, is a multidomain adapter protein that links a variety of signaling proteins and ion channels to the dystrophin protein complex. Without syntrophin, utrophin and neuronal nitric oxide synthase mu (nNOSmu) fail to localize to the NMJ and the AChRs are distributed abnormally. Here we investigate the contribution of syntrophin domains to AChR distribution and to localization of utrophin and nNOSmu at the NMJ. Transgenic mice expressing alpha-syntrophin lacking portions of the first pleckstrin homology (PH) domain (DeltaPH1a or DeltaPH1b) or the entire PDZ domain (DeltaPDZ) were bred onto the alpha-syntrophin null background. As expected the DeltaPDZ transgene did not restore the NMJ localization of nNOS. The DeltaPH1a transgene did restore postsynaptic nNOS but surprisingly did not restore sarcolemmal nNOS (although sarcolemmal aquaporin-4 was restored). Mice lacking the alpha-syntrophin PDZ domain or either half of the PH1 domain were able to restore utrophin to the NMJ but did not correct the aberrant AChR distribution of the alpha-syntrophin knock-out mice. However, mice expressing both the transgenic DeltaPDZ and the transgenic DeltaPH1a constructs did restore normal AChR distribution, demonstrating that both domains are required but need not be confined within the same protein to function. We conclude that the PH1 and PDZ domains of alpha-syntrophin work in concert to facilitate the localization of AChRs and nNOS at the NMJ.
Figures
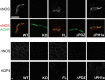
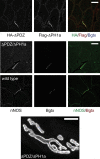
References
-
- Adams ME, Tesch Y, Percival JM, Albrecht DE, Conhaim JI, Anderson K, Froehner SC. Differential targeting of nNOS and AQP4 to dystrophin-deficient sarcolemma by membrane-directed alpha-dystrobrevin. J Cell Sci. 2008;121:48–54. - PubMed
-
- Alessi A, Bragg AD, Percival JM, Yoo J, Albrecht DE, Froehner SC, Adams ME. gamma-Syntrophin scaffolding is spatially and functionally distinct from that of the alpha/beta syntrophins. Exp Cell Res. 2006;312:3084–3095. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
