Role of aberrant striatal dopamine D1 receptor/cAMP/protein kinase A/DARPP32 signaling in the paradoxical calming effect of amphetamine
- PMID: 20720111
- PMCID: PMC6633484
- DOI: 10.1523/JNEUROSCI.1682-10.2010
Role of aberrant striatal dopamine D1 receptor/cAMP/protein kinase A/DARPP32 signaling in the paradoxical calming effect of amphetamine
Abstract
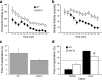
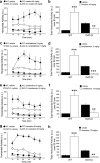
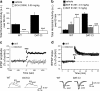
Attention deficit/hyperactivity disorder (ADHD) is characterized by inattention, impulsivity, and motor hyperactivity. Several lines of research support a crucial role for the dopamine transporter (DAT) gene in this psychiatric disease. Consistently, the most commonly prescribed medications in ADHD treatment are stimulant drugs, known to preferentially act on DAT. Recently, a knock-in mouse [DAT-cocaine insensitive (DAT-CI)] has been generated carrying a cocaine-insensitive DAT that is functional but with reduced dopamine uptake function. DAT-CI mutants display enhanced striatal extracellular dopamine levels and basal motor hyperactivity. Herein, we showed that DAT-CI animals present higher striatal dopamine turnover, altered basal phosphorylation state of dopamine and cAMP-regulated phosphoprotein 32 kDa (DARPP32) at Thr75 residue, but preserved D(2) receptor (D(2)R) function. However, although we demonstrated that striatal D(1) receptor (D(1)R) is physiologically responsive under basal conditions, its stimulus-induced activation strikingly resulted in paradoxical electrophysiological, behavioral, and biochemical responses. Indeed, in DAT-CI animals, (1) striatal LTP was completely disrupted, (2) R-(+)-6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide (SKF 81297) treatment induced paradoxical motor calming effects, and (3) SKF 81297 administration failed to increase cAMP/protein kinase A (PKA)/DARPP32 signaling. Such biochemical alteration selectively affected dopamine D(1)Rs since haloperidol, by blocking the tonic inhibition of D(2)R, unmasked a normal activation of striatal adenosine A(2A) receptor-mediated cAMP/PKA/DARPP32 cascade in mutants. Most importantly, our studies highlighted that amphetamine, nomifensine, and bupropion, through increased striatal dopaminergic transmission, are able to revert motor hyperactivity of DAT-CI animals. Overall, our results suggest that the paradoxical motor calming effect induced by these drugs in DAT-CI mutants depends on selective aberrant phasic activation of D(1)R/cAMP/PKA/DARPP32 signaling in response to increased striatal extracellular dopamine levels.
Figures






Similar articles
-
Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice.J Biol Chem. 2006 Oct 27;281(43):32072-80. doi: 10.1074/jbc.M606062200. Epub 2006 Sep 5. J Biol Chem. 2006. PMID: 16954211
-
Selective Manipulation of G-Protein γ7 Subunit in Mice Provides New Insights into Striatal Control of Motor Behavior.J Neurosci. 2021 Nov 3;41(44):9065-9081. doi: 10.1523/JNEUROSCI.1211-21.2021. Epub 2021 Sep 20. J Neurosci. 2021. PMID: 34544837 Free PMC article.
-
Amphetamine activates Rho GTPase signaling to mediate dopamine transporter internalization and acute behavioral effects of amphetamine.Proc Natl Acad Sci U S A. 2015 Dec 22;112(51):E7138-47. doi: 10.1073/pnas.1511670112. Epub 2015 Nov 9. Proc Natl Acad Sci U S A. 2015. PMID: 26553986 Free PMC article.
-
Genetic targeting of the amphetamine and methylphenidate-sensitive dopamine transporter: on the path to an animal model of attention-deficit hyperactivity disorder.Neurochem Int. 2014 Jul;73:56-70. doi: 10.1016/j.neuint.2013.11.009. Epub 2013 Dec 8. Neurochem Int. 2014. PMID: 24332984 Free PMC article. Review.
-
Caffeine as a psychomotor stimulant: mechanism of action.Cell Mol Life Sci. 2004 Apr;61(7-8):857-72. doi: 10.1007/s00018-003-3269-3. Cell Mol Life Sci. 2004. PMID: 15095008 Free PMC article. Review.
Cited by
-
A2A adenosine receptor antagonism enhances synaptic and motor effects of cocaine via CB1 cannabinoid receptor activation.PLoS One. 2012;7(6):e38312. doi: 10.1371/journal.pone.0038312. Epub 2012 Jun 8. PLoS One. 2012. PMID: 22715379 Free PMC article.
-
Animal models to guide clinical drug development in ADHD: lost in translation?Br J Pharmacol. 2011 Oct;164(4):1107-28. doi: 10.1111/j.1476-5381.2011.01412.x. Br J Pharmacol. 2011. PMID: 21480864 Free PMC article. Review.
-
The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders.Br J Pharmacol. 2011 Oct;164(4):1301-21. doi: 10.1111/j.1476-5381.2011.01323.x. Br J Pharmacol. 2011. PMID: 21410459 Free PMC article. Review.
-
ALDH1A1 regulates postsynaptic μ-opioid receptor expression in dorsal striatal projection neurons and mitigates dyskinesia through transsynaptic retinoic acid signaling.Sci Rep. 2019 Mar 5;9(1):3602. doi: 10.1038/s41598-019-40326-x. Sci Rep. 2019. PMID: 30837649 Free PMC article.
-
Dopamine transporter mutant animals: a translational perspective.J Neurogenet. 2016 Mar;30(1):5-15. doi: 10.3109/01677063.2016.1144751. J Neurogenet. 2016. PMID: 27276191 Free PMC article. Review.
References
-
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51:87–96. - PubMed
-
- Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J Biol Chem. 2006;281:32072–32080. - PubMed
-
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study “recognition memory.”. Nat Protoc. 2006;1:1306–1311. - PubMed
-
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
