Functional role of gangliotetraosylceramide in epithelial-to-mesenchymal transition process induced by hypoxia and by TGF-{beta}
- PMID: 20720159
- PMCID: PMC2992377
- DOI: 10.1096/fj.10-162107
Functional role of gangliotetraosylceramide in epithelial-to-mesenchymal transition process induced by hypoxia and by TGF-{beta}
Abstract
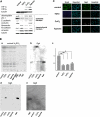
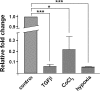
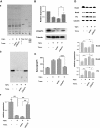
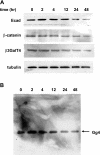
The epithelial-to-mesenchymal transition (EMT) is a basic cellular process that plays a key role in normal embryonic development and in cancer progression/metastasis. Our previous study indicated that EMT processes of mouse and human epithelial cells induced by TGF-β display clear reduction of gangliotetraosylceramide (Gg4) and ganglioside GM2, suggesting a close association of glycosphingolipids (GSLs) with EMT. In the present study, using normal murine mammary gland (NMuMG) cells, we found that levels of Gg4 and of mRNA for the UDP-Gal:β1-3galactosyltransferase-4 (β3GalT4) gene, responsible for reduction of Gg4, were reduced in EMT induced by hypoxia (∼1% O(2)) or CoCl(2) (hypoxia mimic), similarly to that for TGF-β-induced EMT. An increase in the Gg4 level by its exogenous addition or by transfection of the β3GalT4 gene inhibited the hypoxia-induced or TGF-β-induced EMT process, including changes in epithelial cell morphology, enhanced motility, and associated changes in epithelial vs. mesenchymal molecules. We also found that Gg4 is closely associated with E-cadherin and β-catenin. These results suggest that the β3GalT4 gene, responsible for Gg4 expression, is down-regulated in EMT; and Gg4 has a regulatory function in the EMT process in NMuMG cells, possibly through interaction with epithelial molecules important to maintain epithelial cell membrane organization.
Figures
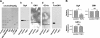




Similar articles
-
Downregulation of gangliotetraosylceramide and β1,3-galactosyltransferase-4 gene expression by Smads during transforming growth factor β-induced epithelial-mesenchymal transition.Mol Med Rep. 2015 Mar;11(3):2241-7. doi: 10.3892/mmr.2014.2912. Epub 2014 Nov 10. Mol Med Rep. 2015. PMID: 25384497
-
Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines.Proc Natl Acad Sci U S A. 2009 May 5;106(18):7461-6. doi: 10.1073/pnas.0902368106. Epub 2009 Apr 20. Proc Natl Acad Sci U S A. 2009. PMID: 19380734 Free PMC article.
-
MIR-99a and MIR-99b modulate TGF-β induced epithelial to mesenchymal plasticity in normal murine mammary gland cells.PLoS One. 2012;7(1):e31032. doi: 10.1371/journal.pone.0031032. Epub 2012 Jan 27. PLoS One. 2012. PMID: 22299047 Free PMC article.
-
Involvement of O-glycosylation defining oncofetal fibronectin in epithelial-mesenchymal transition process.Proc Natl Acad Sci U S A. 2011 Oct 25;108(43):17690-5. doi: 10.1073/pnas.1115191108. Epub 2011 Oct 17. Proc Natl Acad Sci U S A. 2011. PMID: 22006308 Free PMC article.
-
Sweet and sour: the impact of differential glycosylation in cancer cells undergoing epithelial-mesenchymal transition.Front Oncol. 2014 Mar 25;4:59. doi: 10.3389/fonc.2014.00059. eCollection 2014. Front Oncol. 2014. PMID: 24724053 Free PMC article. Review.
Cited by
-
Glycosylation in Cancer: Interplay between Multidrug Resistance and Epithelial-to-Mesenchymal Transition?Front Oncol. 2016 Jun 22;6:158. doi: 10.3389/fonc.2016.00158. eCollection 2016. Front Oncol. 2016. PMID: 27446804 Free PMC article. Review.
-
Role of Glycans in Cancer Cells Undergoing Epithelial-Mesenchymal Transition.Front Oncol. 2016 Feb 17;6:33. doi: 10.3389/fonc.2016.00033. eCollection 2016. Front Oncol. 2016. PMID: 26925388 Free PMC article. Review.
-
Hypoxia stimulates the EMT of gastric cancer cells through autocrine TGFβ signaling.PLoS One. 2013 May 17;8(5):e62310. doi: 10.1371/journal.pone.0062310. Print 2013. PLoS One. 2013. PMID: 23690936 Free PMC article.
-
TGFβ-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer.Cell Mol Life Sci. 2020 Jun;77(11):2103-2123. doi: 10.1007/s00018-019-03398-6. Epub 2019 Dec 10. Cell Mol Life Sci. 2020. PMID: 31822964 Free PMC article. Review.
-
Bisecting N-Acetylglucosamine on EGFR Inhibits Malignant Phenotype of Breast Cancer via Down-Regulation of EGFR/Erk Signaling.Front Oncol. 2020 Jun 16;10:929. doi: 10.3389/fonc.2020.00929. eCollection 2020. Front Oncol. 2020. PMID: 32612952 Free PMC article.
References
-
- Hay E. D. (2005) The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev. Dyn. 233, 706–720 - PubMed
-
- Sugrue S. P., Hay E. D. (1982) Interaction of embryonic corneal epithelium with exogenous collagen, laminin, and fibronectin: role of endogenous protein synthesis. Dev. Biol. 92, 97–106 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

