Ionic currents in intimal cultured synoviocytes from the rabbit
- PMID: 20720182
- PMCID: PMC2980311
- DOI: 10.1152/ajpcell.00028.2010
Ionic currents in intimal cultured synoviocytes from the rabbit
Abstract
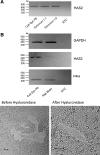
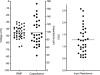
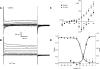
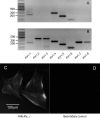
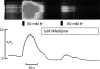
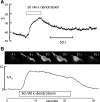
Hyaluronan, a joint lubricant and regulator of synovial fluid content, is secreted by fibroblast-like synoviocytes lining the joint cavity, and secretion is greatly stimulated by Ca(2+)-dependent protein kinase C. This study aimed to define synoviocyte membrane currents and channels that may influence synoviocyte Ca(2+) dynamics. Resting membrane potential ranged from -30 mV to -66 mV (mean -45 ± 8.60 mV, n = 40). Input resistance ranged from 0.54 GΩ to 2.6 GΩ (mean 1.28 ± 0.57 GΩ; ν = 33). Cell capacitance averaged 97.97 ± 5.93 pF. Voltage clamp using C(s+) pipette solution yielded a transient inward current that disappeared in Ca(2+)-free solutions and was blocked by 1 μM nifedipine, indicating an L-type calcium current. The current was increased fourfold by the calcium channel activator FPL 64176 (300 nM). Using K(+) pipette solution, depolarizing steps positive to -40 mV evoked an outward current that showed kinetics and voltage dependence of activation and inactivation typical of the delayed rectifier potassium current. This was blocked by the nonspecific delayed rectifier blocker 4-aminopyridine. The synoviocytes expressed mRNA for four Kv1 subtypes (Kv1.1, Kv1.4, Kv1.5, and Kv1.6). Correolide (1 μM), margatoxin (100 nM), and α-dendrotoxin block these Kv1 subtypes, and all of these drugs significantly reduced synoviocyte outward current. The current was blocked most effectively by 50 nM κ-dendrotoxin, which is specific for channels containing a Kv1.1 subunit, indicating that Kv1.1 is critical, either as a homomultimeric channel or as a component of a heteromultimeric Kv1 channel. When 50 nM κ-dendrotoxin was added to current-clamped synoviocytes, the cells depolarized by >20 mV and this was accompanied by an increase in intracellular calcium concentration. Similarly, depolarization of the cells with high external potassium solution caused an increase in intracellular calcium, and this effect was greatly reduced by 1 μM nifedipine. In conclusion, fibroblast-like synoviocytes cultured from the inner synovium of the rabbit exhibit voltage-dependent inward and outward currents, including Ca(2+) currents. They thus express ion channels regulating membrane Ca(2+) permeability and electrochemical gradient. Since Ca(2+)-dependent kinases are major regulators of synovial hyaluronan secretion, the synoviocyte ion channels are likely to be important in the regulation of hyaluronan secretion.
Figures




References
-
- Aune TM. Inhibition of arthritis by L-type calcium channel antagonists nimodipine, nisoldipine and nifedipine. US patent 5478848, 1995
-
- Bae YM, Kim A, Kim J, Park SW, Kim TK, Lee YR, Kim B, Cho SI. Serotonin depolarizes the membrane potential in rat mesenteric artery myocytes by decreasing voltage-gated K+ currents. Biochem Biophys Res Commun 347: 468–476, 2006 - PubMed
-
- Baumgarten L, Villereal M. Bradykinin stimulates Ca2+ entry via nitrendipine-sensitive Ca2+ channels in cultured human fibroblasts. Agents Actions Suppl 38: 1–8, 1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

