Axonal pathology precedes demyelination in a mouse model of X-linked demyelinating/type I Charcot-Marie Tooth neuropathy
- PMID: 20720503
- PMCID: PMC3034224
- DOI: 10.1097/NEN.0b013e3181efa658
Axonal pathology precedes demyelination in a mouse model of X-linked demyelinating/type I Charcot-Marie Tooth neuropathy
Abstract
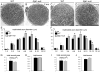
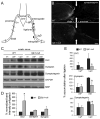
The X-linked demyelinating/type I Charcot-Marie-Tooth neuropathy (CMT1X) is an inherited peripheral neuropathy caused by mutations in GJB1, the gene that encodes the gap junction protein connexin32. Connexin32 is expressed by myelinating Schwann cells and forms gap junctions in noncompact myelin areas, but axonal involvement is more prominent in X-linked compared with other forms of demyelinating Charcot-Marie-Tooth disease. To clarify the cellular and molecular mechanisms of axonal pathology in CMT1X, we studied Gjb1-null mice at early stages (i.e. 2-4 months old) of the neuropathy, when there is minimal or no demyelination. The diameters of large myelinated axons were progressively reduced in Gjb1-null mice compared with those in wild-type littermates. Furthermore, neurofilaments were relatively more dephosphorylated and more densely packed starting at 2 months of age. Increased expression of β-amyloid precursor protein, a marker of axonal damage, was also detected in Gjb1-null nerves. Finally, fast axonal transport, assayed by sciatic nerve ligation experiments, was slower in distal axons of Gjb1-null versus wild-type animals with reduced accumulation of synaptic vesicle-associated proteins. These findings demonstrate that axonal abnormalities including impaired cytoskeletal organization and defects in axonal transport precede demyelination in this mouse model of CMT1X.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

