Solid-phase synthesis of oligodeoxynucleotides containing N4-[2-(t-butyldisulfanyl)ethyl]-5-methylcytosine moieties
- PMID: 20720521
- PMCID: PMC6257692
- DOI: 10.3390/molecules15085692
Solid-phase synthesis of oligodeoxynucleotides containing N4-[2-(t-butyldisulfanyl)ethyl]-5-methylcytosine moieties
Abstract
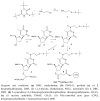
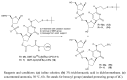
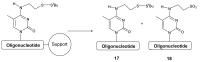
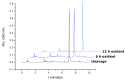
An efficient route for the synthesis of the phosphoramidite derivative of 5-methylcytosine bearing a tert-butylsulfanyl group protected thiol is described. This building block is used for the preparation of oligonucleotides carrying a thiol group at the nucleobase at the internal position of a DNA sequence. The resulting thiolated oligonucleotides are useful intermediates to generate oligonucleotide conjugates carrying molecules of interest at internal positions of a DNA sequence.
Figures


Similar articles
-
Synthesis and characterization of oligonucleotides containing conformationally constrained bicyclo[3.1.0]hexane pseudosugar analogs.Nucleic Acids Res. 2004 Jul 9;32(12):3642-50. doi: 10.1093/nar/gkh667. Print 2004. Nucleic Acids Res. 2004. PMID: 15247346 Free PMC article.
-
Synthesis of a thymine glycol building block and its incorporation into oligonucleotides.Nucleic Acids Symp Ser. 2000;(44):121-2. doi: 10.1093/nass/44.1.121. Nucleic Acids Symp Ser. 2000. PMID: 12903298
-
[Synthesis and fluorescent properties of 5-(1-pyrenylethynyl)-2'- deoxyuridine-containing oligodeoxynucleotides].Bioorg Khim. 2000 Jan;26(1):39-50. Bioorg Khim. 2000. PMID: 10806551 Russian.
-
Chemical synthesis of 2'-deoxyoligonucleotides containing 5-fluoro-2'-deoxycytidine.Nucleic Acids Res. 1992 May 25;20(10):2421-6. doi: 10.1093/nar/20.10.2421. Nucleic Acids Res. 1992. PMID: 1598200 Free PMC article.
-
The 3-(N-tert-butylcarboxamido)-1-propyl group as an attractive phosphate/thiophosphate protecting group for solid-phase oligodeoxyribonucleotide synthesis.J Org Chem. 2002 Sep 6;67(18):6430-8. doi: 10.1021/jo0258608. J Org Chem. 2002. PMID: 12201764
Cited by
-
Synthesis of oligonucleotides carrying thiol groups using a simple reagent derived from threoninol.Molecules. 2012 Aug 24;17(9):10026-45. doi: 10.3390/molecules170910026. Molecules. 2012. PMID: 22922274 Free PMC article.
-
Improved Metal-Free Approach for the Synthesis of Protected Thiol Containing Thymidine Nucleoside Phosphoramidite and Its Application for the Synthesis of Ligatable Oligonucleotide Conjugates.Pharmaceutics. 2023 Jan 11;15(1):248. doi: 10.3390/pharmaceutics15010248. Pharmaceutics. 2023. PMID: 36678876 Free PMC article.
-
Functionalization and self-assembly of DNA bidimensional arrays.Int J Mol Sci. 2011;12(9):5641-51. doi: 10.3390/ijms12095641. Epub 2011 Sep 2. Int J Mol Sci. 2011. PMID: 22016615 Free PMC article.
-
Thioctic acid derivatives as building blocks to incorporate DNA oligonucleotides onto gold nanoparticles.Molecules. 2014 Jul 18;19(7):10495-523. doi: 10.3390/molecules190710495. Molecules. 2014. PMID: 25045890 Free PMC article.
References
-
- Beaucage S.L., Iyer R.P. The functionalization of oligonucleotide via phosphoramidite derivatives. Tetrahedron. 1993;49:1925–1963. doi: 10.1016/S0040-4020(01)86295-5. - DOI
-
- Corey D.R. 48000-fold Acceleration of hybridization by chemically modified oligonucleotides. J. Am. Chem. Soc. 1995;117:9373–9374. doi: 10.1021/ja00141a038. - DOI
-
- Eritja R., Pons A., Escarceller M., Giralt E., Albericio F. Synthesis of defined peptide-oligonucleotide hybrids containing a nuclear transport signal sequence. Tetrahedron. 1991;47:4113–4120. doi: 10.1016/S0040-4020(01)86448-6. - DOI
-
- Zuckermann R.N., Schultz P.G. A hybrid sequence selective ribonuclease S. J. Am. Chem. Soc. 1988;110:6592–6594. doi: 10.1021/ja00227a066. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

