Solid-phase synthesis of oligodeoxynucleotides containing N4-[2-(t-butyldisulfanyl)ethyl]-5-methylcytosine moieties
- PMID: 20720521
- PMCID: PMC6257692
- DOI: 10.3390/molecules15085692
Solid-phase synthesis of oligodeoxynucleotides containing N4-[2-(t-butyldisulfanyl)ethyl]-5-methylcytosine moieties
Abstract
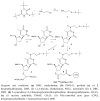
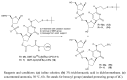
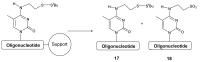
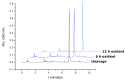
An efficient route for the synthesis of the phosphoramidite derivative of 5-methylcytosine bearing a tert-butylsulfanyl group protected thiol is described. This building block is used for the preparation of oligonucleotides carrying a thiol group at the nucleobase at the internal position of a DNA sequence. The resulting thiolated oligonucleotides are useful intermediates to generate oligonucleotide conjugates carrying molecules of interest at internal positions of a DNA sequence.
Figures


References
-
- Beaucage S.L., Iyer R.P. The functionalization of oligonucleotide via phosphoramidite derivatives. Tetrahedron. 1993;49:1925–1963. doi: 10.1016/S0040-4020(01)86295-5. - DOI
-
- Corey D.R. 48000-fold Acceleration of hybridization by chemically modified oligonucleotides. J. Am. Chem. Soc. 1995;117:9373–9374. doi: 10.1021/ja00141a038. - DOI
-
- Eritja R., Pons A., Escarceller M., Giralt E., Albericio F. Synthesis of defined peptide-oligonucleotide hybrids containing a nuclear transport signal sequence. Tetrahedron. 1991;47:4113–4120. doi: 10.1016/S0040-4020(01)86448-6. - DOI
-
- Zuckermann R.N., Schultz P.G. A hybrid sequence selective ribonuclease S. J. Am. Chem. Soc. 1988;110:6592–6594. doi: 10.1021/ja00227a066. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

