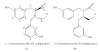Flaxseed ingestion alters ratio of enterolactone enantiomers in human serum
- PMID: 20721350
- PMCID: PMC2915798
- DOI: 10.1155/2010/403076
Flaxseed ingestion alters ratio of enterolactone enantiomers in human serum
Abstract
Enterolactone (EL) is an enterolignan found in human subjects. In this pilot study, the enantiomeric ratios of serum EL were determined in serum from healthy adults during consumption of habitual diet, and after an 8-day supplementation with flaxseed (25 g/day). (-)EL dominated in all serum samples collected during habitual diet consumption. However, the ratio of (-)EL and (+)EL enantiomers differed markedly between individuals. Flaxseed ingestion increased significantly the proportion of (+)EL in all subjects. Moreover, a small but significant increase in serum (-)EL concentration was measured. After flaxseed ingestion, (-)EL concentrations correlated with those of (+)EL suggesting that the stereochemistry of the parent plant lignan in flaxseed is not a major determinant of EL formation in human subjects. Comparison of EL concentrations obtained with the validated chromatographic methods (HPLC-MS/MS, HPLC-CEAD, and GC-MS) and the time-resolved fluoroimmunoassay (TR-FIA) revealed that the immunoassay method underestimates human serum EL concentrations after the flaxseed ingestion.
Figures
Similar articles
-
Short communication: Concentrations of the mammalian lignan enterolactone in preovulatory follicles and the correlation with intrafollicular estradiol in dairy cows fed extruded flaxseed.J Dairy Sci. 2015 Dec;98(12):8814-7. doi: 10.3168/jds.2015-9699. Epub 2015 Oct 9. J Dairy Sci. 2015. PMID: 26454291
-
The interaction of monensin and flaxseed hulls on ruminal and milk concentration of the mammalian lignan enterolactone in late-lactating dairy cows.J Dairy Res. 2009 Nov;76(4):475-82. doi: 10.1017/S0022029909990215. J Dairy Res. 2009. PMID: 19825214 Clinical Trial.
-
Human metabolism of mammalian lignan precursors in raw and processed flaxseed.Am J Clin Nutr. 1999 Mar;69(3):549-55. doi: 10.1093/ajcn/69.3.549. Am J Clin Nutr. 1999. PMID: 10075344 Clinical Trial.
-
Flaxseed influences urinary lignan excretion in a dose-dependent manner in postmenopausal women.Cancer Epidemiol Biomarkers Prev. 2000 Oct;9(10):1113-8. Cancer Epidemiol Biomarkers Prev. 2000. PMID: 11045796 Clinical Trial.
-
Assessing exposure to lignans and their metabolites in humans.J AOAC Int. 2006 Jul-Aug;89(4):1174-81. J AOAC Int. 2006. PMID: 16915861 Review.
Cited by
-
Evolving concepts: how diet and the intestinal microbiome act as modulators of breast malignancy.ISRN Oncol. 2013 Sep 25;2013:693920. doi: 10.1155/2013/693920. ISRN Oncol. 2013. PMID: 24187630 Free PMC article. Review.
-
Extraction Techniques and Analytical Methods for Isolation and Characterization of Lignans.Plants (Basel). 2022 Sep 5;11(17):2323. doi: 10.3390/plants11172323. Plants (Basel). 2022. PMID: 36079704 Free PMC article. Review.
-
Enterolactone Induces G1-phase Cell Cycle Arrest in Nonsmall Cell Lung Cancer Cells by Downregulating Cyclins and Cyclin-dependent Kinases.Nutr Cancer. 2017 May-Jun;69(4):652-662. doi: 10.1080/01635581.2017.1296169. Epub 2017 Mar 21. Nutr Cancer. 2017. PMID: 28323486 Free PMC article.
-
Metabolomics Insights into Chemical Convergence in Xanthomonas perforans and Metabolic Changes Following Treatment with the Small Molecule Carvacrol.Metabolites. 2021 Dec 16;11(12):879. doi: 10.3390/metabo11120879. Metabolites. 2021. PMID: 34940636 Free PMC article.
References
-
- Heinonen S, Nurmi T, Liukkonen K, et al. In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. Journal of Agricultural and Food Chemistry. 2001;49(7):3178–3186. - PubMed
-
- Ingram D, Sanders K, Kolybaba M, Lopez D. Case-control study of phyto-oestrogens and breast cancer. Lancet. 1997;350(9083):990–994. - PubMed
-
- Pietinen P, Stumpf K, Männistö S, Kataja V, Uusitupa M, Adlercreutz H. Serum enterolactone and risk of breast cancer: a case-control study in Eastern Finland. Cancer Epidemiology Biomarkers and Prevention. 2001;10(4):339–344. - PubMed
-
- Vanharanta M, Voutilainen S, Lakka TA, van der Lee M, Adlercreutz H, Salonen JT. Risk of acute coronary events according to serum concentrations of enterolactone: a prospective population-based case-control study. Lancet. 1999;354(9196):2112–2115. - PubMed
-
- Vanharanta M, Voutilainen S, Rissanen TH, Adlercreutz H, Salonen JT. Risk of cardiovascular disease-related and all-cause death according to serum concentrations of enterolactone: Kuopio ischaemic heart disease risk factor study. Archives of Internal Medicine. 2003;163(9):1099–1104. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous