A futility study of minocycline in Huntington's disease
- PMID: 20721920
- PMCID: PMC8801051
- DOI: 10.1002/mds.23236
A futility study of minocycline in Huntington's disease
Abstract
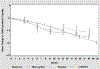
This study assessed the futility of proceeding with a Phase 3 clinical trial of minocycline as a disease-modifying treatment for Huntington's disease (HD). One hundred fourteen research participants with HD were randomized, 87 to minocycline (200 mg/d) and 27 to placebo. The change in Total Functional Capacity (TFC) score from baseline to Mo 18 was prespecified as the primary measure of HD progression. By using a futility design, we tested the null hypothesis that minocycline would reduce the mean decline in TFC score by at least 25% compared to a fixed value obtained from a historical database, with a one-tailed significance level of 10%. The placebo group was included to facilitate blinding. Rejection of the null hypothesis would discourage a major definitive trial of minocycline in HD. For the primary analysis, missing data were handled by carrying forward the last available observation; a secondary analysis used multiple imputations. The mean TFC decline in the minocycline group was 1.55 (SD 1.85), and futility was not declared (P = 0.12) for the primary analysis. When multiple imputation was used to handle missing data, the mean TFC decline in the minocycline group of 1.71 (SD 1.96, P = 0.07) suggested futility, as was the case for prespecified secondary outcome measures. There were no safety abnormalities attributable to minocycline. Based on the threshold of 25% improvement in TFC, further study of minocycline 200 mg/d in HD was not warranted. Futility designs aid in screening potential therapies for HD.
Conflict of interest statement
Potential conflict of interest: The authors have no personal conflicts of interest related to the study or study drug. There was no ghost writing.
Figures
References
-
- Marshall F, Shoulson I. Huntington’s disease: clinical features and therapy. In: Watts R, Kollers W, editors. Movement disorders: neurologic principles and practice. New York: McGraw HIll; 1997. p.491–502.
-
- Zhu S, Stavrovskiaya I, Drozda M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 2002;417:74–78. - PubMed
-
- Chen M, Ona C, Li M, et al. Minocycline inhibits caspase-1 andcaspase-2 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature Med 2000;6:797–801. - PubMed
-
- Stack E, Smith K, Ryu H, et al. Combination therapy using minocycline and coenzyme Q10 in R6/2 transgenic Huntington’s disease mice. Biochim Biophys Acta 2006;1762:373–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical