Cycling through metabolism
- PMID: 20721988
- PMCID: PMC3118222
- DOI: 10.1002/emmm.201000089
Cycling through metabolism
Abstract
Since the discovery of cyclins, the role of cell cycle regulators in the control of cell proliferation has been extensively studied. It is clear that proliferation requires an adapted metabolic response of the cells; hence the regulation of cell cycle must be linked to metabolic control. While at a much slower pace, the impact that the activities of cell cycle regulators such as cyclins, cyclin dependent kinases or E2F factor, transcription factor have on cell metabolism are also being uncovered. Here we will focus on recent data implicating cell cycle regulators in metabolic control, with particular attention to studies performed using mouse models. Furthermore, we will discuss the possible relevance of these findings in the context of metabolic disorders such as obesity or diabetes.
Figures


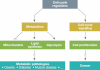
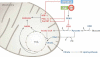

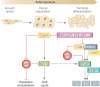
References
-
- Abella A, Dubus P, Malumbres M, Rane SG, Kiyokawa H, Sicard A, Vignon F, Langin D, Barbacid M, Fajas L. Cdk4 promotes adipogenesis through PPARgamma activation. Cell Metab. 2005;2:239–249. - PubMed
-
- Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudiere E, Foretz M, Viollet B, et al. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab. 2007;5:476–487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

