Intraarticular injection of heparin-binding insulin-like growth factor 1 sustains delivery of insulin-like growth factor 1 to cartilage through binding to chondroitin sulfate
- PMID: 20722014
- PMCID: PMC2994951
- DOI: 10.1002/art.27709
Intraarticular injection of heparin-binding insulin-like growth factor 1 sustains delivery of insulin-like growth factor 1 to cartilage through binding to chondroitin sulfate
Abstract
Objective: Insulin-like growth factor 1 (IGF-1) stimulates cartilage repair but is not a practical therapy due to its short half-life. We have previously modified IGF-1 by adding a heparin-binding domain and have shown that this fusion protein (HB-IGF-1) stimulates sustained proteoglycan synthesis in cartilage. This study was undertaken to examine the mechanism by which HB-IGF-1 is retained in cartilage and to test whether HB-IGF-1 provides sustained growth factor delivery to cartilage in vivo and to human cartilage explants.
Methods: Retention of HB-IGF-1 and IGF-1 was analyzed by Western blotting. The necessity of heparan sulfate (HS) or chondroitin sulfate (CS) glycosaminoglycans (GAGs) for binding was tested using enzymatic removal and cells with genetic deficiency of HS. Binding affinities of HB-IGF-1 and IGF-1 proteins for isolated GAGs were examined by surface plasmon resonance and enzyme-linked immunosorbent assay.
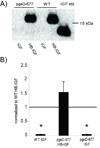
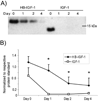
Results: In cartilage explants, chondroitinase treatment decreased binding of HB-IGF-1, whereas heparitinase had no effect. Furthermore, HS was not necessary for HB-IGF-1 retention on cell monolayers. Binding assays showed that HB-IGF-1 bound both CS and HS, whereas IGF-1 did not bind either. After intraarticular injection in rat knees, HB-IGF-1 was retained in articular and meniscal cartilage, but not in tendon, consistent with enhanced delivery to CS-rich cartilage. Finally, HB-IGF-1 was retained in human cartilage explants but IGF-1 was not.
Conclusion: Our findings indicate that after intraarticular injection in rats, HB-IGF-1 is specifically retained in cartilage through its high abundance of CS. Modification of growth factors with heparin-binding domains may be a new strategy for sustained and specific local delivery to cartilage.
Copyright © 2010 by the American College of Rheumatology.
Figures



References
-
- Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Practice & Research Clinical Rheumatology. 2006;20:1003–1025. - PubMed
-
- Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19:11–17. - PubMed
-
- Hui W, Rowan AD, Cawston T. Modulation of the expression of matrix metalloproteinase and tissue inhibitors of metalloproteinases by TGF-β1 and IGF-1 in primary human articular and bovine nasal chondrocytes stimulated with TNF-α. Cytokine. 2001;16:31–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

