Genome-based characterization of two prenylation steps in the assembly of the stephacidin and notoamide anticancer agents in a marine-derived Aspergillus sp
- PMID: 20722388
- PMCID: PMC2941195
- DOI: 10.1021/ja1049302
Genome-based characterization of two prenylation steps in the assembly of the stephacidin and notoamide anticancer agents in a marine-derived Aspergillus sp
Abstract
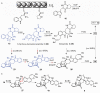
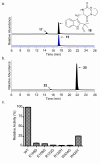
Stephacidin and notoamide natural products belong to a group of prenylated indole alkaloids containing a core bicyclo[2.2.2]diazaoctane ring system. These bioactive fungal secondary metabolites have a range of unusual structural and stereochemical features but their biosynthesis has remained uncharacterized. Herein, we report the first biosynthetic gene cluster for this class of fungal alkaloids based on whole genome sequencing of a marine-derived Aspergillus sp. Two central pathway enzymes catalyzing both normal and reverse prenyltransfer reactions were characterized in detail. Our results establish the early steps for creation of the prenylated indole alkaloid structure and suggest a scheme for the biosynthesis of stephacidin and notoamide metabolites. The work provides the first genetic and biochemical insights for understanding the structural diversity of this important family of fungal alkaloids.
Figures


References
-
- Keller NP, Turner G, Bennett JW. Nat Rev Microbiol. 2005;3(12):937–47. - PubMed
-
- Pelaez F. Biological activities of fungal metabolites. In: An Z, editor. Handbook of Industrial Mycology. Marcel Dekker; New York: 2005. pp. 49–922.
-
- Williams RM, Cox RJ. Acc Chem Res. 2003;36(2):127–39. - PubMed
-
- Stocking EM, Sanz-Cervera JF, Williams RM. Angew Chem Int Ed Engl. 2001;40(7):1296–1298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

