Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update
- PMID: 20722720
- PMCID: PMC2943059
- DOI: 10.1111/j.1460-9568.2010.07354.x
Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update
Abstract
A first step towards understanding the operation of a neural network is identification of the populations of neurons that contribute to it. Our aim here is to reassess the basis for subdivision of adult mammalian spinal interneurons that mediate reflex actions from tendon organs (group Ib afferents) and muscle spindle secondary endings (group II afferents) into separate populations. Re-examining the existing experimental data, we find no compelling reasons to consider intermediate zone interneurons with input from group Ib afferents to be distinct from those co-excited by group II afferents. Similar patterns of distributed input have been found in subpopulations that project ipsilaterally, contralaterally or bilaterally, and in both excitatory and inhibitory interneurons; differences in input from group I and II afferents to individual interneurons showed intra- rather than inter-population variation. Patterns of reflex actions evoked from group Ib and II afferents and task-dependent changes in these actions, e.g. during locomotion, may likewise be compatible with mediation by premotor interneurons integrating information from both group I and II afferents. Pathological changes after injuries of the central nervous system in humans and the lineage of different subclasses of embryonic interneurons may therefore be analyzed without need to consider subdivision of adult intermediate zone interneurons into subpopulations with group Ib or group II input. We propose renaming these neurons 'group I/II interneurons'.
© 2010 The Authors. European Journal of Neuroscience © 2010 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures
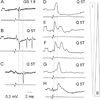
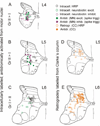

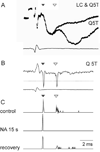
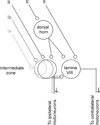
References
-
- Al-Mosawie A, Wilson JM, Brownstone RM. Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur. J. Neurosci. 2007;26:3003–3015. - PubMed
-
- Alstermark B, Kummel H. Transneuronal transport of wheat germ agglutinin conjugated horseradish peroxidase into last order spinal interneurones projecting to acromio- and spinodeltoideus motoneurones in the cat. 1. Location of labelled interneurones and influence of synaptic activity on the transneuronal transport. Exp. Brain Res. 1990;80:83–95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

