Two percent topical phenytoin sodium solution in treating pyoderma gangrenosum: a cohort study
- PMID: 20722769
- PMCID: PMC7951286
- DOI: 10.1111/j.1742-481X.2010.00725.x
Two percent topical phenytoin sodium solution in treating pyoderma gangrenosum: a cohort study
Abstract
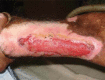
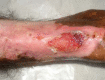
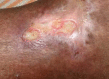
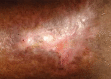
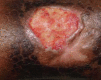
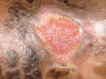
Oral phenytoin is an extensively used medicine for the treatment of convulsive disorders. Topical phenytoin has also been used for various types of ulcers. To determine the effectiveness of 2% topical phenytoin sodium solution in treating recalcitrant pyoderma gangrenosum. Six patients with treatment-resistant pyoderma gangrenosum who attended to Dermatology Unit/Ward were taken to the study and applied topical 2% phenytoin sodium solution to the wounds alone with other systemic therapy. Response to the treatment was assessed weekly. Three patients had idiopathic PG and other three had secondary diseases. At the end of the 4th week four patients showed complete resolution of the ulcers whereas other two patients showed the partial resolution. No adverse effects were noted. Phenytoin sodium 2% solution is beneficial for pyoderma gangrenosum (PG) with various etiologies. It enhanced the healing of the ulcer especially when the patient has treatment resistant disease.
© 2010 The Authors. Journal Compilation © 2010 Blackwell Publishing Ltd and Medicalhelplines.com Inc.
Figures
Similar articles
-
Treatment of pyoderma gangrenosum with topical factor XIII.Eur J Dermatol. 2013 Sep-Oct;23(5):653-7. doi: 10.1684/ejd.2013.2147. Eur J Dermatol. 2013. PMID: 24135449
-
Topical treatment with 1% sodium cromoglycate in pyoderma gangrenosum.Dermatology. 1996;192(3):252-4. doi: 10.1159/000246377. Dermatology. 1996. PMID: 8726641
-
Clinical outcomes and response of patients applying topical therapy for pyoderma gangrenosum: A prospective cohort study.J Am Acad Dermatol. 2016 Nov;75(5):940-949. doi: 10.1016/j.jaad.2016.06.016. Epub 2016 Aug 5. J Am Acad Dermatol. 2016. PMID: 27502313
-
Delayed diagnosis of pyoderma gangrenosum: a case study.Ostomy Wound Manage. 2008 Nov;54(11):32-6. Ostomy Wound Manage. 2008. PMID: 19037135 Review.
-
A novel topical therapy for resistant and early peristomal pyoderma gangrenosum.Int Wound J. 2019 Oct;16(5):1136-1143. doi: 10.1111/iwj.13164. Epub 2019 Jul 12. Int Wound J. 2019. PMID: 31298491 Free PMC article. Review.
Cited by
-
Pyoderma Gangrenosum as a Presenting Feature of Undifferentiated Spondyloarthropathy with Erosive Inflammatory Arthritis.Case Rep Rheumatol. 2020 Mar 26;2020:1848562. doi: 10.1155/2020/1848562. eCollection 2020. Case Rep Rheumatol. 2020. PMID: 32274238 Free PMC article.
-
Pyoderma Gangrenosum: An Updated Literature Review on Established and Emerging Pharmacological Treatments.Am J Clin Dermatol. 2022 Sep;23(5):615-634. doi: 10.1007/s40257-022-00699-8. Epub 2022 May 24. Am J Clin Dermatol. 2022. PMID: 35606650 Free PMC article. Review.
-
Topical phenytoin for treating pressure ulcers.Cochrane Database Syst Rev. 2017 Feb 22;2(2):CD008251. doi: 10.1002/14651858.CD008251.pub2. Cochrane Database Syst Rev. 2017. PMID: 28225152 Free PMC article.
-
Pyoderma gangrenosum: An update.Indian Dermatol Online J. 2012 Jan;3(1):7-13. doi: 10.4103/2229-5178.93482. Indian Dermatol Online J. 2012. PMID: 23130252 Free PMC article.
-
Pyoderma gangrenosum: From historical perspectives to emerging investigations.Int Wound J. 2020 Oct;17(5):1255-1265. doi: 10.1111/iwj.13389. Epub 2020 May 6. Int Wound J. 2020. PMID: 32378319 Free PMC article. Review.
References
-
- Bolognia JL, Jorizzo JL, Rapini RP. Neutrophilic dermatoses. Dermatology. Vol 2 . St. Louis: Mosby, 2008:383–84.
-
- Bolognia JL, Jorizzo JL, Rapini RP. Neutrophilic dermatoses. Dermatology. Vol 2 . St. Louis: Mosby, 2008:386.
-
- Powell FC, Collins S. Pyoderma gangernosum. Clin Dermatol 2000;18:283–93. - PubMed
-
- Duffill MB. Cyclosporine, azathiorine and local therapy for pyoderma gangrenosum. Australas J Dermatol 2007;35:15–8. - PubMed
-
- Newell LM, Malkinson FD. Pyoderma gangrenosum cyclophosphamide therapy. Arch Dermatol 1983;119:495–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

