Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia
- PMID: 20722971
- PMCID: PMC2970698
- DOI: 10.1111/j.1471-4159.2010.06946.x
Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia
Abstract
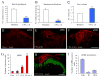
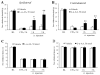
Activation of extracellular signal-regulated kinase (ERK) in spinal cord neurons could serve as a marker for sensitization of dorsal horn neurons in persistent pain. ERK is normally activated by high-threshold noxious stimuli. We investigated how low-threshold mechanical stimuli could activate ERK after complete Freund's adjuvant (CFA)-induced inflammation. Unilateral injection of CFA induced ipsilateral heat hyperalgesia and bilateral mechanical allodynia. CFA-induced ERK activation in ipsilateral dorsal horn neurons declined after 2 days. Interestingly, low-threshold mechanical stimulation given by light touch either on the inflamed paw or the contralateral non-inflamed paw dramatically increased ERK phosphorylation in the dorsal horn ipsilateral to touch stimulation. Notably, light touch induced ERK phosphorylation mainly in superficial neurons in laminae I-IIo. Intrathecal administration of the astroglial toxin L-α-aminoadipate on post-CFA day 2 reversed CFA-induced bilateral mechanical allodynia but not heat hyperalgesia. Furthermore, L-α-aminoadipate, the glial inhibitor fluorocitrate, and a peptide inhibitor of c-Jun N-terminal Kinase all reduced light touch-evoked ERK activation ipsilateral to touch. Collectively, these data suggest that (i) ERK can be activated in superficial dorsal horn neurons by low-threshold mechanical stimulation under pathological condition and (ii) ERK activation by light touch is associated with mechanical allodynia and requires an astrocyte network.
© 2010 The Authors. Journal Compilation © 2010 International Society for Neurochemistry.
Figures




Similar articles
-
Recent advances in our understanding of the organization of dorsal horn neuron populations and their contribution to cutaneous mechanical allodynia.J Neural Transm (Vienna). 2020 Apr;127(4):505-525. doi: 10.1007/s00702-020-02159-1. Epub 2020 Apr 2. J Neural Transm (Vienna). 2020. PMID: 32239353 Free PMC article. Review.
-
The c-Jun N-terminal kinase 1 (JNK1) in spinal astrocytes is required for the maintenance of bilateral mechanical allodynia under a persistent inflammatory pain condition.Pain. 2010 Feb;148(2):309-319. doi: 10.1016/j.pain.2009.11.017. Pain. 2010. PMID: 20022176 Free PMC article.
-
ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity.J Neurosci. 2002 Jan 15;22(2):478-85. doi: 10.1523/JNEUROSCI.22-02-00478.2002. J Neurosci. 2002. PMID: 11784793 Free PMC article.
-
Inflammation enhances Y1 receptor signaling, neuropeptide Y-mediated inhibition of hyperalgesia, and substance P release from primary afferent neurons.Neuroscience. 2014 Jan 3;256:178-94. doi: 10.1016/j.neuroscience.2013.10.054. Epub 2013 Oct 31. Neuroscience. 2014. PMID: 24184981 Free PMC article.
-
Activation of JNK pathway in persistent pain.Neurosci Lett. 2008 Jun 6;437(3):180-3. doi: 10.1016/j.neulet.2008.03.017. Epub 2008 Mar 13. Neurosci Lett. 2008. PMID: 18455869 Free PMC article. Review.
Cited by
-
Contribution of microglia and astrocytes to the central sensitization, inflammatory and neuropathic pain in the juvenile rat.Mol Pain. 2012 Jun 15;8:43. doi: 10.1186/1744-8069-8-43. Mol Pain. 2012. PMID: 22703840 Free PMC article.
-
N-methyl-D-aspartate Receptor Subunits 2A and 2B Mediate Connexins and Pannexins in the Trigeminal Ganglion Involved in Orofacial Inflammatory Allodynia during Temporomandibular Joint Inflammation.Mol Neurobiol. 2025 Jan;62(1):1247-1265. doi: 10.1007/s12035-024-04291-5. Epub 2024 Jul 8. Mol Neurobiol. 2025. PMID: 38976127
-
Chronic tooth pulp inflammation induces persistent expression of phosphorylated ERK (pERK) and phosphorylated p38 (pp38) in trigeminal subnucleus caudalis.Neuroscience. 2014 Jun 6;269(100):318-30. doi: 10.1016/j.neuroscience.2014.03.056. Epub 2014 Apr 5. Neuroscience. 2014. PMID: 24709040 Free PMC article.
-
Gp120 in the pathogenesis of human immunodeficiency virus-associated pain.Ann Neurol. 2014 Jun;75(6):837-50. doi: 10.1002/ana.24139. Epub 2014 May 28. Ann Neurol. 2014. PMID: 24633867 Free PMC article.
-
Recent advances in our understanding of the organization of dorsal horn neuron populations and their contribution to cutaneous mechanical allodynia.J Neural Transm (Vienna). 2020 Apr;127(4):505-525. doi: 10.1007/s00702-020-02159-1. Epub 2020 Apr 2. J Neural Transm (Vienna). 2020. PMID: 32239353 Free PMC article. Review.
References
-
- Adwanikar H, Karim F, Gereau RWt. Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain. 2004;111:125–135. - PubMed
-
- Ambalavanar R, Dessem D, Moutanni A, Yallampalli C, Yallampalli U, Gangula P, Bai G. Muscle inflammation induces a rapid increase in calcitonin gene-related peptide (CGRP) mRNA that temporally relates to CGRP immunoreactivity and nociceptive behavior. Neuroscience. 2006;143:875–884. - PubMed
-
- Baron R. Neuropathic pain: a clinical perspective. Handbook of experimental pharmacology. 2009:3–30. - PubMed
-
- Bertorelli R, Corradini L, Rafiq K, Tupper J, Calo G, Ongini E. Nociceptin and the ORL-1 ligand [Phe1psi (CH2-NH)Gly2]nociceptin(1-13)NH2 exert anti-opioid effects in the Freund’s adjuvant-induced arthritic rat model of chronic pain. British journal of pharmacology. 1999;128:1252–1258. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

