Occurrence of specific humoral non-responsiveness to swine antigens following administration of GalT-KO bone marrow to baboons
- PMID: 20723202
- PMCID: PMC2942069
- DOI: 10.1111/j.1399-3089.2010.00600.x
Occurrence of specific humoral non-responsiveness to swine antigens following administration of GalT-KO bone marrow to baboons
Abstract
Background: Hematopoietic chimerism induces transplantation tolerance across allogeneic and xenogeneic barriers, but has been difficult to achieve in the pig-to-primate model. We have now utilized swine with knockout of the gene coding for alpha-1,3-galactosyltransferase (GalT-KO pigs) as bone marrow donors in an attempt to achieve chimerism and tolerance by avoiding the effects of natural antibodies to Gal determinants on pig hematopoietic cells.
Methods: Baboons (n = 4; Baboons 1 to 4 = B156, B158, B167, and B175, respectively) were splenectomized and conditioned with TBI (150 cGy), thymic irradiation (700 cGy), T cell depletion with rabbit anti-thymocyte globulin (rATG) and rat anti-primate CD2 (LoCD2b), and received FK506 and supportive therapy for 28 days. All animals received GalT-KO bone marrow (1 to 2 x 10(9) cells/kg) in two fractions on days 0 and 2, and were thereafter monitored for the presence of pig cells by flow cytometry, for porcine progenitor cells by PCR of BM colony-forming units, and for cellular reactivity to pig cells by mixed lymphocyte reaction (MLR). In vitro antibody formation to LoCD2b and rATG was tested by ELISA; antibody reactivity to GalT-KO pig cells was tested by flow cytometry and cytotoxicity assays. Additionally, Baboons 3 and 4 received orthotopic kidney transplants on days 17 and 2, respectively, to test the potential impact of the protocol on renal transplantation.
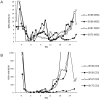
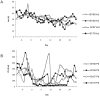
Results: None of the animals showed detectable pig cells by flow cytometry for more than 12 h post-BM infusion. However, porcine progenitor cell engraftment, as evidenced by pig-derived colony forming units in the BM, as well as peripheral microchimerism in the thymus, lymph node, and peripheral blood was detected by PCR in baboons 1 and 2 for at least 28 days post-transplant. ELISA results confirmed humoral immunocompetence at time of transplantation as antibody titers to rat (LoCD2b) and rabbit (ATG) increased within 2 weeks. However, no induced antibodies to GalT-KO pig cells or increased donor specific cytotoxicity was detectable by flow cytometry. In contrast, baboons 3 and 4 developed serum antibodies to pig cells as well as to rat and rabbit immunoglobulin by day 14. Retrospective analysis revealed that although all four baboons possessed low levels of antibody-mediated cytotoxicity to GalT-KO cells prior to transplantation, the two baboons (3 and 4) that became sensitized to pig cells (and rejected pig kidneys) had relatively high pre-transplantation titers of anti-non-Gal IgG detectable by flow cytometry, whereas baboons 1 and 2 had undetectable titers.
Conclusions: Engraftment and specific non-responsiveness to pig cells has been achieved in two of four baboons following GalT-KO pig-to-baboon BMT. Engraftment correlated with absence of preformed anti-non-Gal IgG serum antibodies. These results are encouraging with regard to the possibility of achieving transplantation tolerance across this xenogeneic barrier.
(c) 2010 John Wiley & Sons A/S.
Figures




Similar articles
-
Increased levels of anti-non-Gal IgG following pig-to-baboon bone marrow transplantation correlate with failure of engraftment.Xenotransplantation. 2013 Nov-Dec;20(6):458-68. doi: 10.1111/xen.12065. Epub 2013 Oct 29. Xenotransplantation. 2013. PMID: 24289469 Free PMC article.
-
Bone marrow transplantation from alpha1,3-galactosyltransferase gene-knockout pigs in baboons.Xenotransplantation. 2004 Jul;11(4):361-70. doi: 10.1111/j.1399-3089.2004.00151.x. Xenotransplantation. 2004. PMID: 15196131
-
Anti-Gal(alpha)1-3Gal antibody response to porcine bone marrow in unmodified baboons and baboons conditioned for tolerance induction.Transplantation. 1998 Jul 27;66(2):176-82. doi: 10.1097/00007890-199807270-00006. Transplantation. 1998. PMID: 9701260
-
Gal/non-Gal antigens in pig tissues and human non-Gal antibodies in the GalT-KO era.Xenotransplantation. 2011 Jul-Aug;18(4):215-28. doi: 10.1111/j.1399-3089.2011.00644.x. Xenotransplantation. 2011. PMID: 21848538 Review.
-
Histopathology of xenografts in pig to non-human primate discordant xenotransplantation.Clin Transplant. 2010 Jul;24 Suppl 22:11-5. doi: 10.1111/j.1399-0012.2010.01270.x. Clin Transplant. 2010. PMID: 20590687 Review.
Cited by
-
Tolerance in xenotransplantation.Curr Opin Organ Transplant. 2017 Dec;22(6):522-528. doi: 10.1097/MOT.0000000000000466. Curr Opin Organ Transplant. 2017. PMID: 28937406 Free PMC article. Review.
-
Xenotransplantation: immunological hurdles and progress toward tolerance.Immunol Rev. 2014 Mar;258(1):241-58. doi: 10.1111/imr.12152. Immunol Rev. 2014. PMID: 24517437 Free PMC article. Review.
-
Transient-mixed Chimerism With Nonmyeloablative Conditioning Does Not Induce Liver Allograft Tolerance in Nonhuman Primates.Transplantation. 2020 Aug;104(8):1580-1590. doi: 10.1097/TP.0000000000003263. Transplantation. 2020. PMID: 32732835 Free PMC article.
-
Current progress in xenogeneic tolerance.Curr Opin Organ Transplant. 2012 Apr;17(2):168-73. doi: 10.1097/MOT.0b013e32835090f6. Curr Opin Organ Transplant. 2012. PMID: 22262105 Free PMC article. Review.
-
Prolonged Survival of Pig Skin on Baboons After Administration of Pig Cells Expressing Human CD47.Transplantation. 2017 Feb;101(2):316-321. doi: 10.1097/TP.0000000000001267. Transplantation. 2017. PMID: 27232934 Free PMC article.
References
-
- Sachs DH, Sykes M, Robson M, Cooper DK. Xenotransplantation. Adv Immunol. 2001;79:129–223. - PubMed
-
- Tseng YL, Sachs DH, Cooper DK. Porcine hematopoietic progenitor cell transplantation in nonhuman primates: a review of progress. Transplantation. 2005;79:1–9. - PubMed
-
- Lee LA, Sergio JJ, Sykes M. Natural killer cells weakly resist engraftment of allogeneic, long-term, multilineage-repopulating hematopoietic stem cells. Transplantation. 1996;61:125–132. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

