Serotonin synthesis, release and reuptake in terminals: a mathematical model
- PMID: 20723248
- PMCID: PMC2942809
- DOI: 10.1186/1742-4682-7-34
Serotonin synthesis, release and reuptake in terminals: a mathematical model
Abstract
Background: Serotonin is a neurotransmitter that has been linked to a wide variety of behaviors including feeding and body-weight regulation, social hierarchies, aggression and suicidality, obsessive compulsive disorder, alcoholism, anxiety, and affective disorders. Full understanding of serotonergic systems in the central nervous system involves genomics, neurochemistry, electrophysiology, and behavior. Though associations have been found between functions at these different levels, in most cases the causal mechanisms are unknown. The scientific issues are daunting but important for human health because of the use of selective serotonin reuptake inhibitors and other pharmacological agents to treat disorders in the serotonergic signaling system.
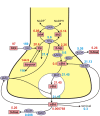
Methods: We construct a mathematical model of serotonin synthesis, release, and reuptake in a single serotonergic neuron terminal. The model includes the effects of autoreceptors, the transport of tryptophan into the terminal, and the metabolism of serotonin, as well as the dependence of release on the firing rate. The model is based on real physiology determined experimentally and is compared to experimental data.
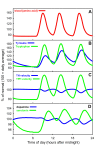
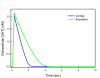
Results: We compare the variations in serotonin and dopamine synthesis due to meals and find that dopamine synthesis is insensitive to the availability of tyrosine but serotonin synthesis is sensitive to the availability of tryptophan. We conduct in silico experiments on the clearance of extracellular serotonin, normally and in the presence of fluoxetine, and compare to experimental data. We study the effects of various polymorphisms in the genes for the serotonin transporter and for tryptophan hydroxylase on synthesis, release, and reuptake. We find that, because of the homeostatic feedback mechanisms of the autoreceptors, the polymorphisms have smaller effects than one expects. We compute the expected steady concentrations of serotonin transporter knockout mice and compare to experimental data. Finally, we study how the properties of the the serotonin transporter and the autoreceptors give rise to the time courses of extracellular serotonin in various projection regions after a dose of fluoxetine.
Conclusions: Serotonergic systems must respond robustly to important biological signals, while at the same time maintaining homeostasis in the face of normal biological fluctuations in inputs, expression levels, and firing rates. This is accomplished through the cooperative effect of many different homeostatic mechanisms including special properties of the serotonin transporters and the serotonin autoreceptors. Many difficult questions remain in order to fully understand how serotonin biochemistry affects serotonin electrophysiology and vice versa, and how both are changed in the presence of selective serotonin reuptake inhibitors. Mathematical models are useful tools for investigating some of these questions.
Figures


Similar articles
-
Autoreceptor control of serotonin dynamics.BMC Neurosci. 2020 Sep 23;21(1):40. doi: 10.1186/s12868-020-00587-z. BMC Neurosci. 2020. PMID: 32967609 Free PMC article.
-
Homeostatic mechanisms in dopamine synthesis and release: a mathematical model.Theor Biol Med Model. 2009 Sep 10;6:21. doi: 10.1186/1742-4682-6-21. Theor Biol Med Model. 2009. PMID: 19740446 Free PMC article.
-
Models of dopaminergic and serotonergic signaling.Pharmacopsychiatry. 2010 May;43 Suppl 1:S61-6. doi: 10.1055/s-0030-1252024. Epub 2010 May 17. Pharmacopsychiatry. 2010. PMID: 20480448
-
Understanding the molecular pharmacology of the serotonergic system: using fluoxetine as a model.J Pharm Pharmacol. 2012 Mar;64(3):317-25. doi: 10.1111/j.2042-7158.2011.01384.x. Epub 2011 Nov 18. J Pharm Pharmacol. 2012. PMID: 22309263 Review.
-
Ligands for the investigation of 5-HT autoreceptor function.Brain Res Bull. 2001 Nov 15;56(5):463-9. doi: 10.1016/s0361-9230(01)00628-1. Brain Res Bull. 2001. PMID: 11750791 Review.
Cited by
-
Assessing the effects of N-acetyl cysteine on growth, antioxidant and immune response in tilapia (Oreochromis niloticus) under different regimes of stocking densities.PLoS One. 2024 Sep 30;19(9):e0307212. doi: 10.1371/journal.pone.0307212. eCollection 2024. PLoS One. 2024. PMID: 39348347 Free PMC article.
-
INFLUENCE OF THE SEROTONERGIC SYSTEM POLYMORPHISM ON THE EXPRESSION OF DENTAL ANXIETY.Acta Clin Croat. 2018 Sep;57(3):417-424. doi: 10.20471/acc.2018.57.03.03. Acta Clin Croat. 2018. PMID: 31168173 Free PMC article.
-
Attenuated serotonin transporter association between dorsal raphe and ventral striatum in major depression.Hum Brain Mapp. 2014 Aug;35(8):3857-66. doi: 10.1002/hbm.22442. Epub 2014 Jan 17. Hum Brain Mapp. 2014. PMID: 24443158 Free PMC article.
-
TPH1 A218C polymorphism and temperament in major depression.BMC Psychiatry. 2013 Apr 18;13:118. doi: 10.1186/1471-244X-13-118. BMC Psychiatry. 2013. PMID: 23597148 Free PMC article.
-
Using mathematical models to understand metabolism, genes, and disease.BMC Biol. 2015 Sep 23;13:79. doi: 10.1186/s12915-015-0189-2. BMC Biol. 2015. PMID: 26400419 Free PMC article.
References
-
- Feldman R, Meyer J, Quenzer L. Principles of Neuropharmacology. Sunderland, MA: Sinauer Associates, Inc; 1997.
-
- Harris-Warrick RM, Cohen AH. Serotonin modulates the central pattern generator for locomotion in the isolated lamprey spinal cord. J Exper Biol. 1985;116:27–46. - PubMed
-
- Strecker RE, Thackar MM, Porkka-Heiskanen T, Dauphin LJ, Bjorkum AA, McCarley RW. Behavioral state-related changes of extracellular serotonin concentration in the pedunculopontine tegmental nucleus: a microdialysis study in freely moving animals. Sleep Res Online. 1999;2:21–27. - PubMed
-
- Carley DW, Radulovacki M. Mirtazapine, a mixed-profile serotonin agonist/antagonist, suppresses sleep apnea in the rat. Am J Respir Crit Care Med. 1999;160:1824–1829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

