Effective induction of protective systemic immunity with nasally administered vaccines adjuvanted with IL-1
- PMID: 20723629
- PMCID: PMC2943532
- DOI: 10.1016/j.vaccine.2010.08.006
Effective induction of protective systemic immunity with nasally administered vaccines adjuvanted with IL-1
Erratum in
- Vaccine. 2011 Mar 24;29(15):2821
Abstract
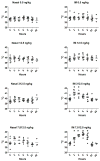
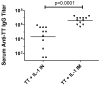
IL-1α and IL-1β were evaluated for their ability to provide adjuvant activity for the induction of serum antibody responses when nasally administered with protein antigens in mice and rabbits. In mice, intranasal (i.n.) immunization with pneumococcal surface protein A (PspA) or tetanus toxoid (TT) combined with IL-1β induced protective immunity that was equivalent to that induced by parenteral immunization. Nasal immunization of awake (i.e., not anesthetized) rabbits with IL-1-adjuvanted vaccines induced highly variable serum antibody responses and was not as effective as parenteral immunization for the induction of antigen-specific serum IgG. However, i.n. immunization of deeply anesthetized rabbits with rPA+IL-1α consistently induced rPA-specific serum IgG ELISA titers that were not significantly different than those induced by intramuscular (IM) immunization with rPA+alum although lethal toxin-neutralizing titers induced by nasal immunization were lower than those induced by IM immunization. Gamma scintigraphy demonstrated that the enhanced immunogenicity of nasal immunization in anesthetized rabbits correlated with an increased nasal retention of i.n. delivered non-permeable radio-labeled colloidal particles. Our results demonstrate that, in mice, IL-1 is an effective adjuvant for nasally administered vaccines for the induction of protective systemic immunity and that in non-rodent species, effective induction of systemic immunity with nasally administered vaccines may require formulations that ensure adequate retention of the vaccine within the nasal cavity.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
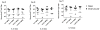
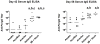

Similar articles
-
A comparison of non-toxin vaccine adjuvants for their ability to enhance the immunogenicity of nasally-administered anthrax recombinant protective antigen.Vaccine. 2013 Mar 1;31(11):1480-9. doi: 10.1016/j.vaccine.2013.01.012. Epub 2013 Jan 23. Vaccine. 2013. PMID: 23352329 Free PMC article.
-
Nasal immunization with anthrax protective antigen protein adjuvanted with polyriboinosinic-polyribocytidylic acid induced strong mucosal and systemic immunities.Pharm Res. 2006 Jun;23(6):1217-26. doi: 10.1007/s11095-006-0206-9. Epub 2006 May 25. Pharm Res. 2006. PMID: 16718616
-
Mucosal immunity elicited by a human-Fcγ receptor-I targeted intranasal vaccine platform enhances resistance against nasopharyngeal colonization of Streptococcus pneumoniae and induces broadly protective immunity against respiratory pathogens.Vaccine. 2025 Feb 27;48:126729. doi: 10.1016/j.vaccine.2025.126729. Epub 2025 Jan 16. Vaccine. 2025. PMID: 39823848
-
Dendritic cell-targeting DNA-based nasal adjuvants for protective mucosal immunity to Streptococcus pneumoniae.Microbiol Immunol. 2017 Jun;61(6):195-205. doi: 10.1111/1348-0421.12487. Microbiol Immunol. 2017. PMID: 28463465 Free PMC article. Review.
-
Interleukin 12 and innate molecules for enhanced mucosal immunity.Immunol Res. 1999;20(3):207-17. doi: 10.1007/BF02790404. Immunol Res. 1999. PMID: 10741861 Review.
Cited by
-
Modified Vaccinia Ankara Virus Vaccination Provides Long-Term Protection against Nasal Rabbitpox Virus Challenge.Clin Vaccine Immunol. 2016 Jul 5;23(7):648-51. doi: 10.1128/CVI.00216-16. Print 2016 Jul. Clin Vaccine Immunol. 2016. PMID: 27146001 Free PMC article.
-
Retention of structure, antigenicity, and biological function of pneumococcal surface protein A (PspA) released from polyanhydride nanoparticles.Acta Biomater. 2013 Sep;9(9):8262-71. doi: 10.1016/j.actbio.2013.06.006. Epub 2013 Jun 14. Acta Biomater. 2013. PMID: 23774257 Free PMC article.
-
In vitro cell culture model of human nasal-associated lymphoid tissue (NALT) to evaluate the humoral immune response to SARS-CoV-2 spike proteins.Saudi J Biol Sci. 2021 Aug;28(8):4516-4521. doi: 10.1016/j.sjbs.2021.04.051. Epub 2021 Apr 24. Saudi J Biol Sci. 2021. PMID: 33942008 Free PMC article.
-
Intranasal mRNA nanoparticle vaccination induces prophylactic and therapeutic anti-tumor immunity.Sci Rep. 2014 Jun 4;4:5128. doi: 10.1038/srep05128. Sci Rep. 2014. PMID: 24894817 Free PMC article.
-
Enhancement of nasal HIV vaccination with adenoviral vector-based nanocomplexes using mucoadhesive and DC-targeting adjuvants.Pharm Res. 2014 Oct;31(10):2748-61. doi: 10.1007/s11095-014-1372-9. Epub 2014 May 3. Pharm Res. 2014. PMID: 24792827
References
-
- Giudice EL, Campbell JD. Needle-free vaccine delivery. Advanced Drug Delivery Reviews. 2006 Apr 20;58(1):68–89. - PubMed
-
- Clements CJ, Larsen G, Jodar L. Technologies that make administration of vaccines safer. Vaccine. 2004 May 7;22(15–16):2054–8. - PubMed
-
- Clements CJ, Aguado MT, Jodar L. Technologies to improve immunisation safety. Drug Saf. 2001;24(14):1019–26. - PubMed
-
- Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Nat Med. 2003;9(1):99. - PubMed
-
- Brody S. Declining HIV rates in Uganda: due to cleaner needles, not abstinence or condoms. Int J STD AIDS. 2004 Jul;15(7):440–1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

