Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial
- PMID: 20723799
- PMCID: PMC3663330
- DOI: 10.1016/j.jacc.2010.03.068
Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial
Abstract
Objectives: The objective was to test the hypothesis that heart failure (HF) patients treated with sertraline will have lower depression scores and fewer cardiovascular events compared with placebo.
Background: Depression is common among HF patients. It is associated with increased hospitalization and mortality.
Methods: The SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial was a randomized, double-blind, placebo-controlled trial of sertraline 50 to 200 mg/day versus matching placebo for 12 weeks. All participants also received nurse-facilitated support. Eligible patients were age 45 years or older with HF (left ventricular ejection fraction < or =45%, New York Heart Association functional class II to IV) and clinical depression (Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition criteria for current major depressive disorder). Those with significant cognitive impairment, psychosis, recent alcohol or drug dependence, bipolar or severe personality disorder, active suicidal ideation, and current antipsychotic or antidepressant medications were excluded. Primary end points were change in depression severity (Hamilton Depression Rating Scale total score) and composite cardiovascular status at 12 weeks.
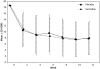
Results: A total of 469 patients were randomized (n = 234 sertraline, n = 235 placebo). The mean +/- SE change from baseline to 12 weeks in the Hamilton Depression Rating Scale total score was -7.1 +/- 0.5 (sertraline) and -6.8 +/- 0.5 (placebo) (p < 0.001 from baseline, p = 0.89 between groups, mean change between groups -0.4; 95% confidence interval: -1.7 to 0.92). The proportions whose composite cardiovascular score worsened, improved, or was unchanged were 29.9%, 40.6%, and 29.5%, respectively, in the sertraline group and 31.1%, 43.8%, and 25.1%, respectively, in the placebo group (p = 0.78).
Conclusions: Sertraline was safe in patients with significant HF. However, treatment with sertraline compared with placebo did not provide greater reduction in depression or improved cardiovascular status among patients with HF and depression. (Antidepressant Medication Treatment for Depression in Individuals With Chronic Heart Failure [SADHART-CHF]; NCT00078286).
Copyright 2010 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Sadness in heart failure: what is a clinician to do?J Am Coll Cardiol. 2010 Aug 24;56(9):700-1. doi: 10.1016/j.jacc.2010.03.067. J Am Coll Cardiol. 2010. PMID: 20723800 No abstract available.
References
-
- Lloyd-Jones D, Adams R, Carnethon M, et al. Heart Disease and Stroke Statistics--2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. - PubMed
-
- Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–442. - PubMed
-
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. - PubMed
-
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–37. - PubMed
-
- Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

