Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction
- PMID: 20723802
- PMCID: PMC2932958
- DOI: 10.1016/j.jacc.2010.03.066
Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction
Abstract
Objectives: The goal of this study was to guide bone marrow-derived human mesenchymal stem cells (hMSCs) into a cardiac progenitor phenotype and assess therapeutic benefit in chronic myocardial infarction.
Background: Adult stem cells, delivered in their naïve state, demonstrate a limited benefit in patients with ischemic heart disease. Pre-emptive lineage pre-specification may optimize therapeutic outcome.
Methods: hMSC were harvested from a coronary artery disease patient cohort. A recombinant cocktail consisting of transforming growth factor-beta(1), bone morphogenetic protein-4, activin A, retinoic acid, insulin-like growth factor-1, fibroblast growth factor-2, alpha-thrombin, and interleukin-6 was formulated to engage hMSC into cardiopoiesis. Derived hMSC were injected into the myocardium of a nude infarcted murine model and followed over 1 year for functional and structural end points.
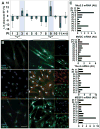
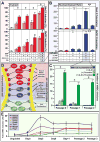
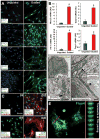
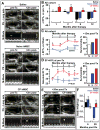
Results: Although the majority of patient-derived hMSC in their native state demonstrated limited effect on ejection fraction, stem cells from rare individuals harbored a spontaneous capacity to improve contractile performance. This reparative cytotype was characterized by high expression of homeobox transcription factor Nkx-2.5, T-box transcription factor TBX5, helix-loop-helix transcription factor MESP1, and myocyte enhancer factor MEF2C, markers of cardiopoiesis. Recombinant cardiogenic cocktail guidance secured the cardiopoietic phenotype across the patient cohort. Compared with unguided counterparts, cardiopoietic hMSC delivered into infarcted myocardium achieved superior functional and structural benefit without adverse side effects. Engraftment into murine hearts was associated with increased human-specific nuclear, sarcomeric, and gap junction content along with induction of myocardial cell cycle activity.
Conclusions: Guided cardiopoiesis thus enhances the therapeutic benefit of bone marrow-derived hMSC in chronic ischemic cardiomyopathy.
Copyright 2010 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest:
A.B., J.B., and A.T. serve on the research advisory board of Cardio3 BioSciences.
Figures




Comment in
-
Boot camp for mesenchymal stem cells.J Am Coll Cardiol. 2010 Aug 24;56(9):735-7. doi: 10.1016/j.jacc.2010.02.064. J Am Coll Cardiol. 2010. PMID: 20723803 Free PMC article. No abstract available.
References
-
- Srivastava D, Ivey KN. Potential of stem-cell-based therapies for heart disease. Nature. 2006;441:1097–9. - PubMed
-
- Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. - PubMed
-
- Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. - PubMed
-
- Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

