Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer's disease
- PMID: 20724290
- PMCID: PMC2948816
- DOI: 10.1093/brain/awq209
Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer's disease
Abstract
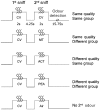
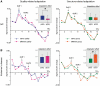
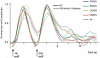
Patients with early-stage Alzheimer's disease exhibit perceptual deficits in odour identification, often before the appearance of overt memory loss. This impairment coincides with the initial accumulation of pathological lesions in limbic olfactory brain regions. Although these data imply that odour stimuli may be effectively used as biological probes of limbic dysfunction, the precise neural mechanisms underlying the olfactory deficits in early Alzheimer's disease remain poorly understood. In the current study, we combined functional magnetic resonance imaging with an olfactory cross-adaptation paradigm to test the hypothesis that perceptual codes of odour quality in posterior piriform cortex are degraded in patients with Alzheimer's disease. In elderly control subjects, sequential presentation of qualitatively similar (versus qualitatively different) odourant pairs elicited cross-adapting responses in posterior piriform cortex, in accord with the pattern observed in healthy young adults. However, this profile was significantly blunted in patients with Alzheimer's disease, reflecting a functional disruption of odour quality coding in this olfactory brain area. These results highlight the potential of olfactory functional magnetic resonance imaging as a non-invasive bioassay of limbic functional integrity, and suggest that such an index could possibly aid in the early diagnosis of Alzheimer's disease. Furthermore, as a putative lesion model of odour quality processing in the human brain, our study suggests a causal role of posterior piriform cortex in differentiating olfactory objects.
Figures




References
-
- Arendt T. Synaptic degeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:167–79. - PubMed
-
- Averback P. Two new lesions in Alzheimer's disease. Lancet. 1983;2:1203. - PubMed
-
- Bacon AW, Bondi MW, Salmon DP, Murphy C. Very early changes in olfactory functioning due to Alzheimer's disease and the role of apolipoprotein E in olfaction. Ann NY Acad Sci USA. 1998;855:723–31. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. - PubMed
-
- Buchsbaum MS, Kesslak JP, Lynch G, Chui H, Wu J, Sicotte N, et al. Temporal and hippocampal metabolic rate during an olfactory memory task assessed by positron emission tomography in patients with dementia of the Alzheimer type and controls. Preliminary studies. Arch Gen Psychiatry. 1991;48:840–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

