Oestrogen prevents cardiomyocyte apoptosis by suppressing p38α-mediated activation of p53 and by down-regulating p53 inhibition on p38β
- PMID: 20724307
- PMCID: PMC3002868
- DOI: 10.1093/cvr/cvq265
Oestrogen prevents cardiomyocyte apoptosis by suppressing p38α-mediated activation of p53 and by down-regulating p53 inhibition on p38β
Abstract
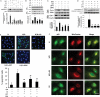
Aims: we have previously shown that 17-β-estradiol (E2) protects cardiomyocytes exposed to simulated ischaemia-reperfusion (I/R) by differentially regulating pro-apoptotic p38α mitogen-activated protein kinase (p38α MAPK) and pro-survival p38β. However, little is known about how E2 modulation of these kinases alters apoptotic signalling. An attractive downstream target is p53, a well-known mediator of apoptosis and a substrate of p38α MAPK. The aim of this study was to determine whether the cytoprotective actions of oestrogen involve regulation of p53 via cardiac p38 MAPKs.
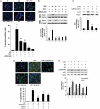
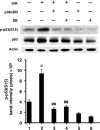
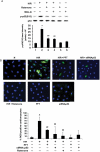
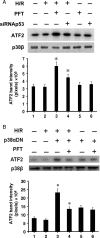
Methods and results: cultured rat cardiomyocytes underwent hypoxia followed by reoxygenation (H/R) to simulate I/R. We found that inhibiting p53 significantly reduced apoptosis. Phosphorylation of p53 at serine 15 [p-p53(S15)] increased after H/R in a p38α MAPK- and reactive oxygen species (ROS)-dependent manner. E2 at 10 nM effectively inhibited p-p53(S15) and mitochondrial translocation of p53. Blocking p53 led to augmented p38β activity and attenuated ROS, suggesting suppression of this antioxidant kinase by p53. The use of a specific agonist for each oestrogen receptor (ER) isoform, ERα and ERβ, demonstrated that both isoforms participate in preventing cell death by inhibiting p53 in the mitochondria-centred apoptotic processes.
Conclusion: our results demonstrate that during H/R stress, cardiomyocytes undergo p53-dependent apoptosis following phosphorylation of p53 by p38α MAPK, leading to p38β suppression. E2 protects cardiomyocytes by inhibiting p38α-p53 signalling in apoptosis.
Figures
Similar articles
-
Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms.J Biol Chem. 2006 Mar 10;281(10):6760-7. doi: 10.1074/jbc.M511024200. Epub 2005 Dec 30. J Biol Chem. 2006. PMID: 16407188
-
Mitochondrial p38β and manganese superoxide dismutase interaction mediated by estrogen in cardiomyocytes.PLoS One. 2014 Jan 22;9(1):e85272. doi: 10.1371/journal.pone.0085272. eCollection 2014. PLoS One. 2014. PMID: 24465521 Free PMC article.
-
Attenuation of mitochondrial and nuclear p38α signaling: a novel mechanism of estrogen neuroprotection in cerebral ischemia.Mol Cell Endocrinol. 2015 Jan 15;400:21-31. doi: 10.1016/j.mce.2014.11.010. Epub 2014 Nov 25. Mol Cell Endocrinol. 2015. PMID: 25462588
-
p38α MAPK pathway: a key factor in colorectal cancer therapy and chemoresistance.World J Gastroenterol. 2014 Aug 7;20(29):9744-58. doi: 10.3748/wjg.v20.i29.9744. World J Gastroenterol. 2014. PMID: 25110412 Free PMC article. Review.
-
Targeting the p38α pathway in chronic inflammatory diseases: Could activation, not inhibition, be the appropriate therapeutic strategy?Pharmacol Ther. 2022 Jul;235:108153. doi: 10.1016/j.pharmthera.2022.108153. Epub 2022 Feb 1. Pharmacol Ther. 2022. PMID: 35121002 Review.
Cited by
-
A novel mechanism by which SDF-1β protects cardiac cells from palmitate-induced endoplasmic reticulum stress and apoptosis via CXCR7 and AMPK/p38 MAPK-mediated interleukin-6 generation.Diabetes. 2013 Jul;62(7):2545-58. doi: 10.2337/db12-1233. Epub 2013 Feb 19. Diabetes. 2013. PMID: 23423573 Free PMC article.
-
p38 MAP kinases in the heart.Gene. 2016 Jan 10;575(2 Pt 2):369-376. doi: 10.1016/j.gene.2015.09.030. Epub 2015 Sep 20. Gene. 2016. PMID: 26390817 Free PMC article. Review.
-
Small molecules, big effects: the role of microRNAs in regulation of cardiomyocyte death.Cell Death Dis. 2014 Jul 17;5(7):e1325. doi: 10.1038/cddis.2014.287. Cell Death Dis. 2014. PMID: 25032848 Free PMC article. Review.
-
A Novel Role of Claudin-5 in Prevention of Mitochondrial Fission Against Ischemic/Hypoxic Stress in Cardiomyocytes.Can J Cardiol. 2021 Oct;37(10):1593-1606. doi: 10.1016/j.cjca.2021.03.021. Epub 2021 Apr 8. Can J Cardiol. 2021. PMID: 33838228 Free PMC article.
-
Association of ESR1 (rs2234693 and rs9340799), CETP (rs708272), MTHFR (rs1801133 and rs2274976) and MS (rs185087) polymorphisms with Coronary Artery Disease (CAD).BMC Cardiovasc Disord. 2020 Jul 18;20(1):340. doi: 10.1186/s12872-020-01618-7. BMC Cardiovasc Disord. 2020. PMID: 32682401 Free PMC article.
References
-
- Shlipak MG, Angeja BG, Go AS, Frederick PD, Canto JG, Grady D. Hormone therapy and in-hospital survival after myocardial infarction in postmenopausal women. Circulation. 2001;104:2300–2304. doi:10.1161/hc4401.98414. - DOI - PubMed
-
- Patten RD, Karas RH. Estrogen replacement and cardiomyocyte protection. Trends Cardiovasc Med. 2006;16:69–75. doi:10.1016/j.tcm.2006.01.002. - DOI - PubMed
-
- Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, et al. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–699. doi:10.1161/01.RES.0000144126.57786.89. - DOI - PubMed
-
- Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem. 2006;281:6760–6767. doi:10.1074/jbc.M511024200. - DOI - PubMed
-
- Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, et al. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J Biol Chem. 1997;272:30122–30128. doi:10.1074/jbc.272.48.30122. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

