Chronic heart failure decreases cross-bridge kinetics in single skeletal muscle fibres from humans
- PMID: 20724360
- PMCID: PMC3000591
- DOI: 10.1113/jphysiol.2010.191957
Chronic heart failure decreases cross-bridge kinetics in single skeletal muscle fibres from humans
Abstract
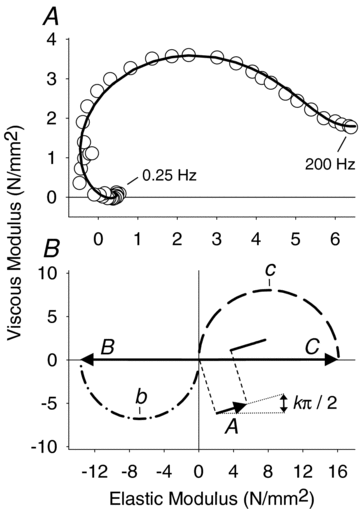
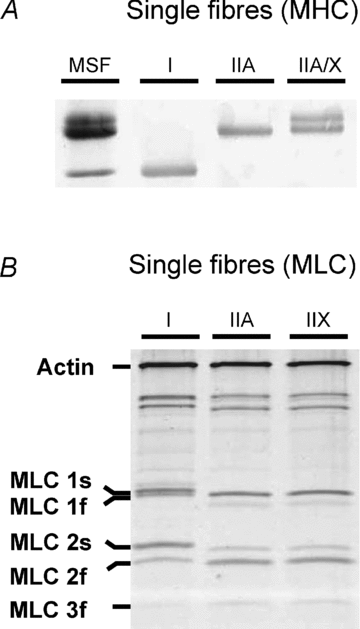
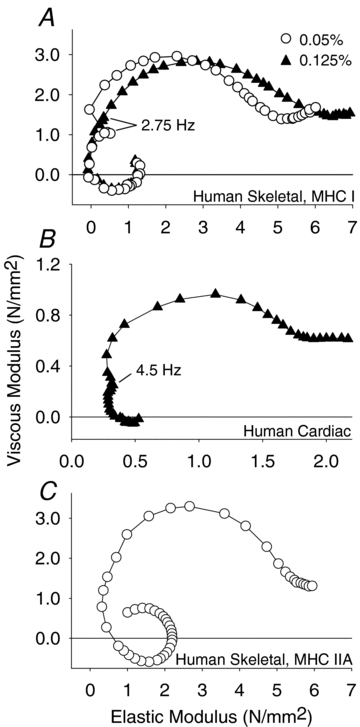
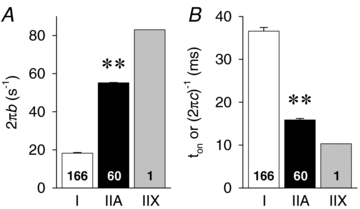
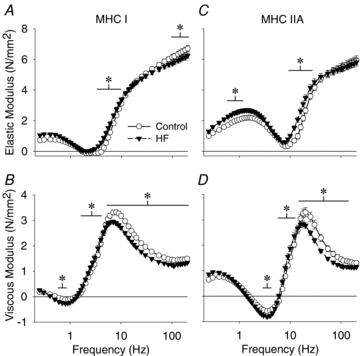
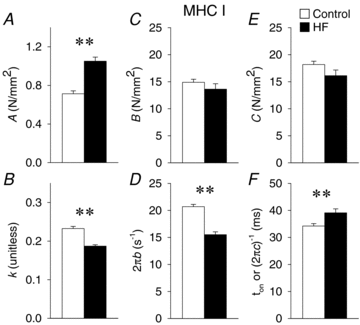
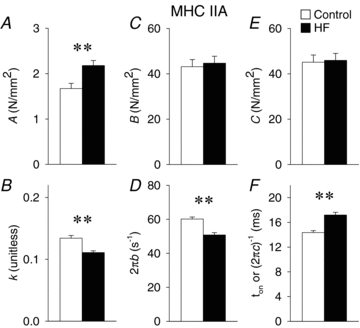
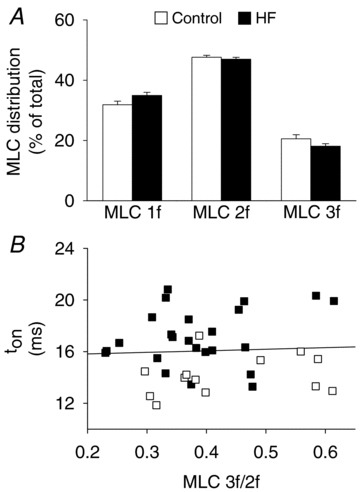
Skeletal muscle function is impaired in heart failure patients due, in part, to loss of myofibrillar protein content, in particular myosin. In the present study, we utilized small-amplitude sinusoidal analysis for the first time in single human skeletal muscle fibres to measure muscle mechanics, including cross-bridge kinetics, to determine if heart failure further impairs contractile performance by altering myofibrillar protein function. Patients with chronic heart failure (n = 9) and controls (n = 6) were recruited of similar age and physical activity to diminish the potentially confounding effects of ageing and muscle disuse. Patients showed decreased cross-bridge kinetics in myosin heavy chain (MHC) I and IIA fibres, partially due to increased myosin attachment time (t(on)). The increased t(on) compensated for myosin protein loss previously found in heart failure patients by increasing the fraction of the total cycle time myosin is bound to actin, resulting in a similar number of strongly bound cross-bridges in patients and controls. Accordingly, isometric tension did not differ between patients and controls in MHC I or IIA fibres. Patients also had decreased calcium sensitivity in MHC IIA fibres and alterations in the viscoelastic properties of the lattice structure of MHC I and IIA fibres. Collectively, these results show that heart failure alters skeletal muscle contraction at the level of the myosin-actin cross-bridge, leading to changes in muscle mechanics which could contribute to impaired muscle function. Additionally, we uncovered a unique kinetic property of MHC I fibres, a potential indication of two distinct populations of cross-bridges, which may have important physiological consequences.
Figures


Similar articles
-
Effect of heart failure on skeletal muscle myofibrillar protein content, isoform expression and calcium sensitivity.Int J Cardiol. 2006 Feb 15;107(2):211-9. doi: 10.1016/j.ijcard.2005.03.024. Int J Cardiol. 2006. PMID: 16412799
-
Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans.J Appl Physiol (1985). 2013 Oct 1;115(7):1004-14. doi: 10.1152/japplphysiol.00563.2013. Epub 2013 Jul 25. J Appl Physiol (1985). 2013. PMID: 23887900 Free PMC article.
-
Mechanisms underlying skeletal muscle weakness in human heart failure: alterations in single fiber myosin protein content and function.Circ Heart Fail. 2009 Nov;2(6):700-6. doi: 10.1161/CIRCHEARTFAILURE.109.876433. Epub 2009 Sep 24. Circ Heart Fail. 2009. PMID: 19919996 Free PMC article.
-
Single muscle fibre contractile function with ageing.J Physiol. 2022 Dec;600(23):5005-5026. doi: 10.1113/JP282298. Epub 2022 Nov 9. J Physiol. 2022. PMID: 36268622 Free PMC article. Review.
-
Muscle mechanics: adaptations with exercise-training.Exerc Sport Sci Rev. 1996;24:427-73. Exerc Sport Sci Rev. 1996. PMID: 8744258 Review.
Cited by
-
Intrinsic skeletal muscle alterations in chronic heart failure patients: a disease-specific myopathy or a result of deconditioning?Heart Fail Rev. 2012 May;17(3):421-36. doi: 10.1007/s10741-011-9289-4. Heart Fail Rev. 2012. PMID: 21996779 Review.
-
Cellular and molecular contractile function in aged human skeletal muscle is altered by phosphate and acidosis and partially reversed with an ATP analog.Am J Physiol Cell Physiol. 2025 Apr 1;328(4):C1220-C1233. doi: 10.1152/ajpcell.00332.2024. Epub 2025 Mar 6. Am J Physiol Cell Physiol. 2025. PMID: 40047118 Free PMC article.
-
Dietary Nitrate and Skeletal Muscle Contractile Function in Heart Failure.Curr Heart Fail Rep. 2016 Aug;13(4):158-65. doi: 10.1007/s11897-016-0293-9. Curr Heart Fail Rep. 2016. PMID: 27271563 Free PMC article. Review.
-
A myosin-based mechanism for stretch activation and its possible role revealed by varying phosphate concentration in fast and slow mouse skeletal muscle fibers.Am J Physiol Cell Physiol. 2019 Dec 1;317(6):C1143-C1152. doi: 10.1152/ajpcell.00206.2019. Epub 2019 Sep 18. Am J Physiol Cell Physiol. 2019. PMID: 31532715 Free PMC article.
-
Influenza Infection has Fiber Type-Specific Effects on Cellular and Molecular Skeletal Muscle Function in Aged Mice.J Gerontol A Biol Sci Med Sci. 2020 Nov 13;75(12):2333-2341. doi: 10.1093/gerona/glaa136. J Gerontol A Biol Sci Med Sci. 2020. PMID: 32492709 Free PMC article.
References
-
- Ades PA, Savage PD, Cress ME, Brochu M, Lee NM, Poehlman ET. Resistance training on physical performance in disabled older female cardiac patients. Med Sci Sports Exerc. 2003;35:1265–1270. - PubMed
-
- Andruchov O, Galler S. Influence of fast and slow alkali myosin light chain isoforms on the kinetics of stretch-induced force transients of fast-twitch type IIA fibres of rat. Pflugers Arch. 2008;455:1165–1172. - PubMed
-
- Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, Fielding RA. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–467. - PubMed
-
- Billeter R, Heizmann CW, Howald H, Jenny E. Analysis of myosin light and heavy chain types in single human skeletal muscle fibers. Eur J Biochem. 1981;116:389–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

