Encoding of whisker input by cerebellar Purkinje cells
- PMID: 20724365
- PMCID: PMC2998225
- DOI: 10.1113/jphysiol.2010.195180
Encoding of whisker input by cerebellar Purkinje cells
Abstract
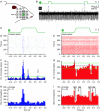
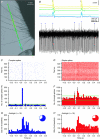
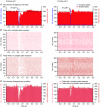
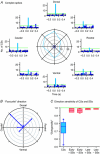
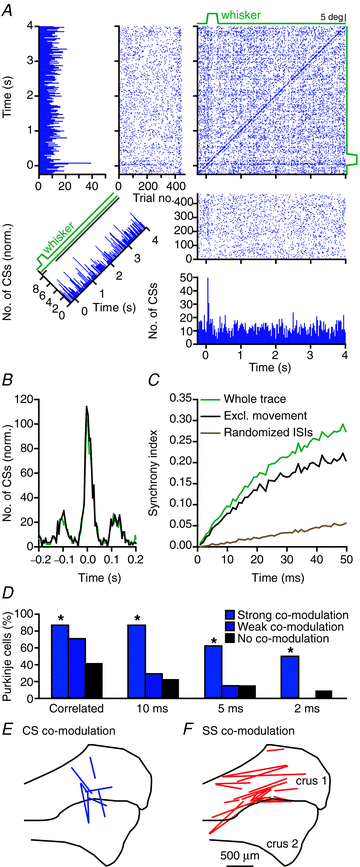
The cerebellar cortex is crucial for sensorimotor integration. Sensorimotor inputs converge on cerebellar Purkinje cells via two afferent pathways: the climbing fibre pathway triggering complex spikes, and the mossy fibre–parallel fibre pathway, modulating the simple spike activities of Purkinje cells. We used, for the first time, the mouse whisker system as a model system to study the encoding of somatosensory input by Purkinje cells.We show that most Purkinje cells in ipsilateral crus 1 and crus 2 of awake mice respond to whisker stimulation with complex spike and/or simple spike responses. Single-whisker stimulation in anaesthetised mice revealed that the receptive fields of complex spike and simple spike responses were strikingly different. Complex spike responses, which proved to be sensitive to the amplitude, speed and direction of whisker movement, were evoked by only one or a few whiskers. Simple spike responses, which were not affected by the direction of movement, could be evoked by many individual whiskers. The receptive fields of Purkinje cells were largely intermingled, and we suggest that this facilitates the rapid integration of sensory inputs from different sources. Furthermore, we describe that individual Purkinje cells, at least under anaesthesia, may be bound in two functional ensembles based on the receptive fields and the synchrony of the complex spike and simple spike responses. The ‘complex spike ensembles’ were oriented in the sagittal plane, following the anatomical organization of the climbing fibres, while the ‘simple spike ensembles’ were oriented in the transversal plane, as are the beams of parallel fibres.
Figures




References
-
- Aertsen AMHJ, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. J Neurophysiol. 1989;61:900–917. - PubMed
-
- Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10:670–681. - PubMed
-
- Armstrong DM, Harvey RJ, Schild RF. Branching of inferior olivary axons to terminate in different folia, lobules or lobes of the cerebellum. Brain Res. 1973a;54:365–371. - PubMed
-
- Armstrong DM, Harvey RJ, Schild RF. The spatial organisation of climbing fibre branching in the cat cerebellum. Exp Brain Res. 1973b;18:40–58. - PubMed
-
- Axelrad H, Crepel F. Représentation sélective des vibrisses mystaciales au niveau des cellules de Purkinje du cervelet par la voie des fibres grimpantes chez le rat. C R Acad Sci Hebd Seances Acad Sci D. 1977;284:1321–1324. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

