A pilot study to assess smokeless tobacco use reduction with varenicline
- PMID: 20724382
- PMCID: PMC2948050
- DOI: 10.1093/ntr/ntq134
A pilot study to assess smokeless tobacco use reduction with varenicline
Abstract
Introduction: Long-term smokeless tobacco (ST) use is known to increase the risk for oropharyngeal cancer, heart attack, and stroke. Extant literature on cigarette smokers suggests that smoking reduction increases smoking abstinence among smokers not interested in quitting. Similarly, a reduction strategy may reduce ST exposure and increase ST abstinence rates among ST users not interested in quitting.
Methods: We conducted a pilot study to obtain preliminary data on the use of 12 weeks of varenicline as a tobacco reduction strategy among ST users not interested in quitting.
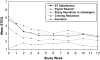
Results: We enrolled 20 male ST users with a mean age of 42.8 ± 11.7 years who used an average of 3.9 ± 1.7 cans/pouches per week for 18.6 ± 8.6 years. At end of treatment (12 weeks), 60% (12/20) of subjects reduced their ST use by ≥ 50% and 15% (3/20) were biochemically confirmed abstinent from tobacco. At end of study (6 months), 50% (10/20) reduced by ≥ 50% of baseline use and 10% (2/20) were biochemically confirmed abstinent from tobacco. Varenicline reduced ST satisfaction, reward, and craving. Among subjects able to reduce ST, all subjects reported that reduction increased motivation and confidence in being able to maintain reduction and quit. The most common side effects were sleep disturbance (25%) and nausea (15%).
Discussion: Varenicline may be effective in reducing ST use and achieving ST abstinence among ST users with no plans to quit but who are interested in reducing their ST use.
Figures
References
-
- Benowitz NL, Ahijevych K, Hall S, Hansson A, Henningfield J, Hurt RD, et al. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. - PubMed
-
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addictive Behaviors. 2007;32:912–923. - PubMed
-
- Faessel HM, Gibbs MA, Clark DJ, Rohrbacher K, Stolar M, Burstein AH. Multiple-dose pharmacokinetics of the selective nicotinic receptor partial agonist, varenicline, in healthy smokers. Journal of Clinical Pharmacology. 2006;46:1439–1448. - PubMed
-
- Falba T, Jofre-Bonet M, Busch S, Duchovny N, Sindelar J. Reduction of quantity smoked predicts future cessation among older smokers. Addiction. 2004;99:93–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical