Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial
- PMID: 20724402
- PMCID: PMC2924475
- DOI: 10.1136/bmj.c4024
Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial
Abstract
Objective: To study rates of relapse in remitted patients with first episode psychosis who either continued or discontinued antipsychotic drugs after at least one year of maintenance treatment.
Design: 12 month randomised, double blind, placebo controlled trial.
Setting: Early psychosis outpatient clinics in Hong Kong.
Participants: 178 patients with first episode psychosis who had received at least one year of antipsychotic drug treatment between September 2003 and July 2006 and had no positive symptoms of psychosis.
Interventions: Patients received either maintenance treatment with quetiapine (400 mg/day) or placebo and were followed up for the next 12 months or until a relapse occurred.
Main outcome measure: Relapse assessed monthly and defined as re-emergence of psychotic symptoms (delusions, conceptual disorganisation, hallucinations, suspiciousness, and unusual thought content) according to predefined thresholds.
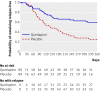
Results: 178 patients were randomised (89 to quetiapine and 89 to placebo). The Kaplan-Meier estimate of the risk of relapse at 12 months was 41% (95% confidence interval 29% to 53%) for the quetiapine group and 79% (68% to 90%) for the placebo group (P<0.001). Although quetiapine was generally well tolerated, the rate of discontinuation due to adverse or serious adverse events was greater in the quetiapine group (18%; 16/89) than in the placebo group (8%; 7/89) (relative risk 2.29, 95% confidence interval 0.99 to 5.28; chi(2)=3.20, df=1; P=0.07).
Conclusion: In a group of asymptomatic patients with first episode psychosis and at least one year of previous antipsychotic drug treatment, maintenance treatment with quetiapine compared with placebo resulted in a substantially lower rate of relapse during the following year. Trial registration Clinical trials NCT00334035.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures

Comment in
-
How long should treatments be continued?BMJ. 2010 Aug 19;341:c4102. doi: 10.1136/bmj.c4102. BMJ. 2010. PMID: 20724403 No abstract available.
References
-
- Robinson DG, Woerner MG, Delman HM, Kane JM. Pharmacological treatments for first-episode schizophrenia. Schizophr Res 2005;31:705-22. - PubMed
-
- Lieberman JA, Jody D, Geisler S, Alvir J, Loebel A, Szymanski S, et al. Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch Gen Psychiatry 1993;50:369-76. - PubMed
-
- Robinson D, Woerner MG, Alvir JMJ, Bilder R, Goldman R, Geisler S, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999;56:241-7. - PubMed
-
- Weiden PJ, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull 1995;21:419-29. - PubMed
-
- Lieberman JA, Alvir JM, Koreen A, Geisler S, Chakos M, Sheitman B, et al. Psychobiologic correlates of treatment response in schizophrenia. Neuropsychopharmacology 1996;14(suppl 3):13-21S. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
